- All Products
- NanoTracers - nanoparticles for SPT
NanoTracers - nanoparticles for SPT
https://www.idylle-labs.com/shop/nanotracers-621 https://www.idylle-labs.com/web/image/product.template/621/image_1920?unique=877a87dFluorescent organic nanoparticles for single-particle tracking
A technology developed by Mireille Blanchard-Desce & Jonathan Daniel (Plateforme NanoMultiPhot, Institut des Sciences Moléculaires de Bordeaux, France)
NanoTracers are a new class of fluorescent tracers obtained from the precipitation of organic dyes in water. Their unique physical and optical properties make them particularly well-suited for single-particle tracking experiments.
What makes them a top choice for single-particle tracking experiments?
Very low tendency to aggregate, thanks to their high intrinsic colloidal stability. This means they don't need any surface modification to prevent aggregation, a very practical feature when working with complex media.
Small size (15 to 30 nm diameter), to achieve finer spatial scales and temporal resolution even in the most crowded environments.
Excellent brightness & photostability, allowing for quick and reliable extraction of diffusion parameters.
Versatile use: naturally biocompatible, these organic nanoparticles come in different versions tailored for specific media (non-biological, intra/extra-cellular or live tissues).
Two types of NanoTracers to fit your desired application:
NanoTracer - Rheo kit
Due to their spontaneous stealth behavior, these nanoparticles do not require the use of antifouling agents to impede interactions with cellular membranes.
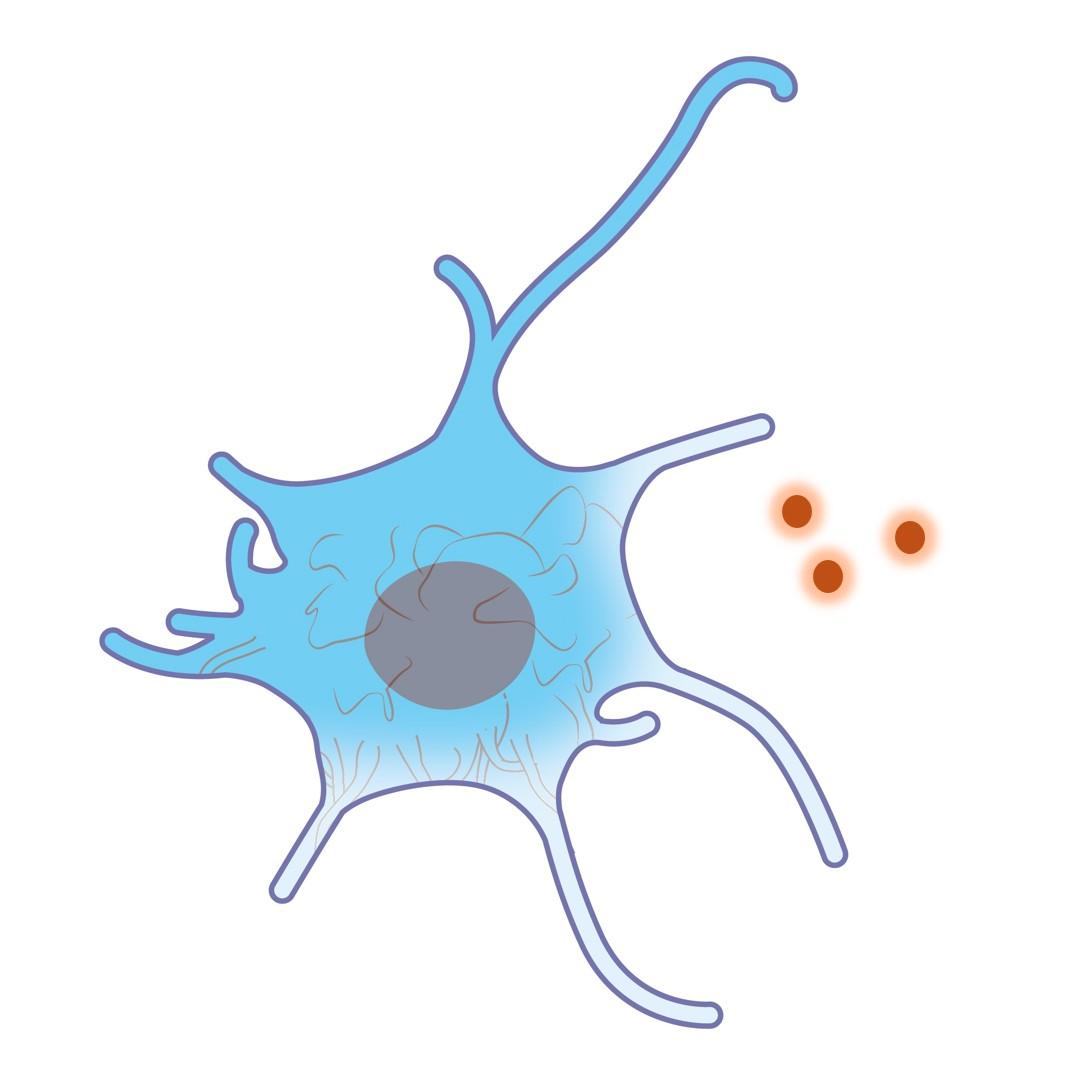
They can be used to assess properties of any type of biological or non-biological media (i.e. extra-cellular space).
With a diameter averaging that of an antibody and record brightness in 2-photon microscopy, they are perfectly compatible with deep tissue imaging for exploring the extra-cellular space of living tissues.
Key features:
- No interactions with living cells
- Red emission
- Compatible with 2-photon microscopy
- Median diameter: 15 nm
NanoTracer - Cell kit
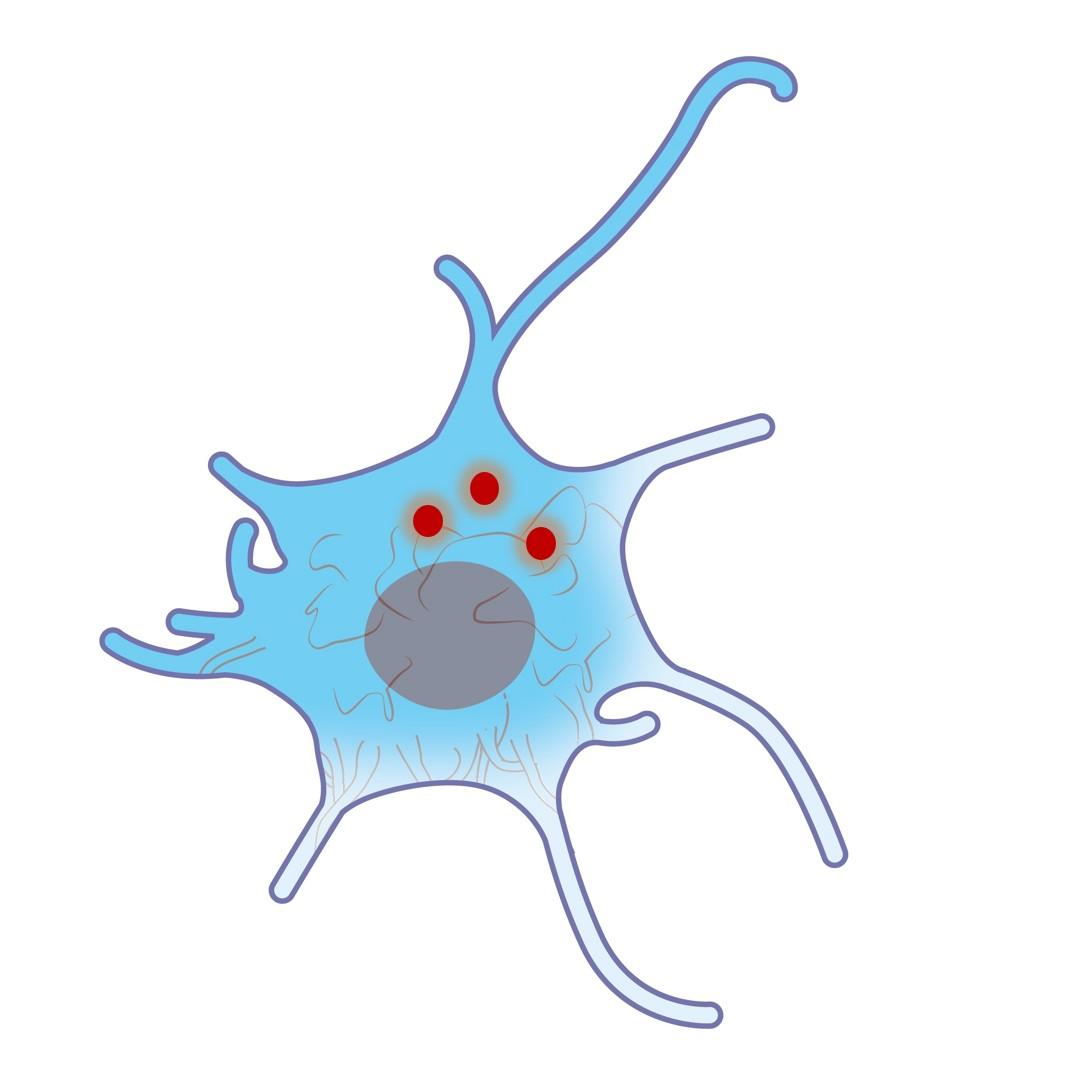
These nanoparticles are naturally uptaken by live cells with no signs of toxicity or unspecific binding to cellular membranes.
They can be used for intracellular single-particle experiments to assess internal cell media properties or track intracellular transport dynamics.
With an ultra-high near-IR brightness and large Stokes' shift, they can be tracked at low laser intensities to best preserve cell physiology.
Key features:
- Natural intracellular uptake
- Bright near-IR emission with minimal laser intensity
- No cytotoxicity
- Compatible with 2-photon microscopy
- Median diameter: 20 nm
Additional documents:
NanoTracer - Rheo kit
- Excitation max: 458 nm
Emission max: 602 nm
Brightness: 1.1 - 6.2 x 10^7 M-1.cm-1 (458nm) - Color: red
- Median diameter: 15 nm or 32 nm
- Concentration: 10^11 particles/mL or 1 nM
- Natural intracellular uptake: no
- Optical parameters under 2-photon excitation:
Excitation max : 840 nm
Cross-section: 1.8 x 10^6 GM
Brightness: 5.4 x 10^5 GM
NanoTracer - Cell kit
- Excitation max: 505 nm
Emission max: 710 nm
Brightness: 1.6x10^6 M-1.cm-1 (505 nm)
2-photon absorption cross-section: 9000 GM - Color: near-IR
- Median diameter: 20 nm
- Concentration: 10^11 particles/mL or 1 nM
- Natural intracellular uptake: yes
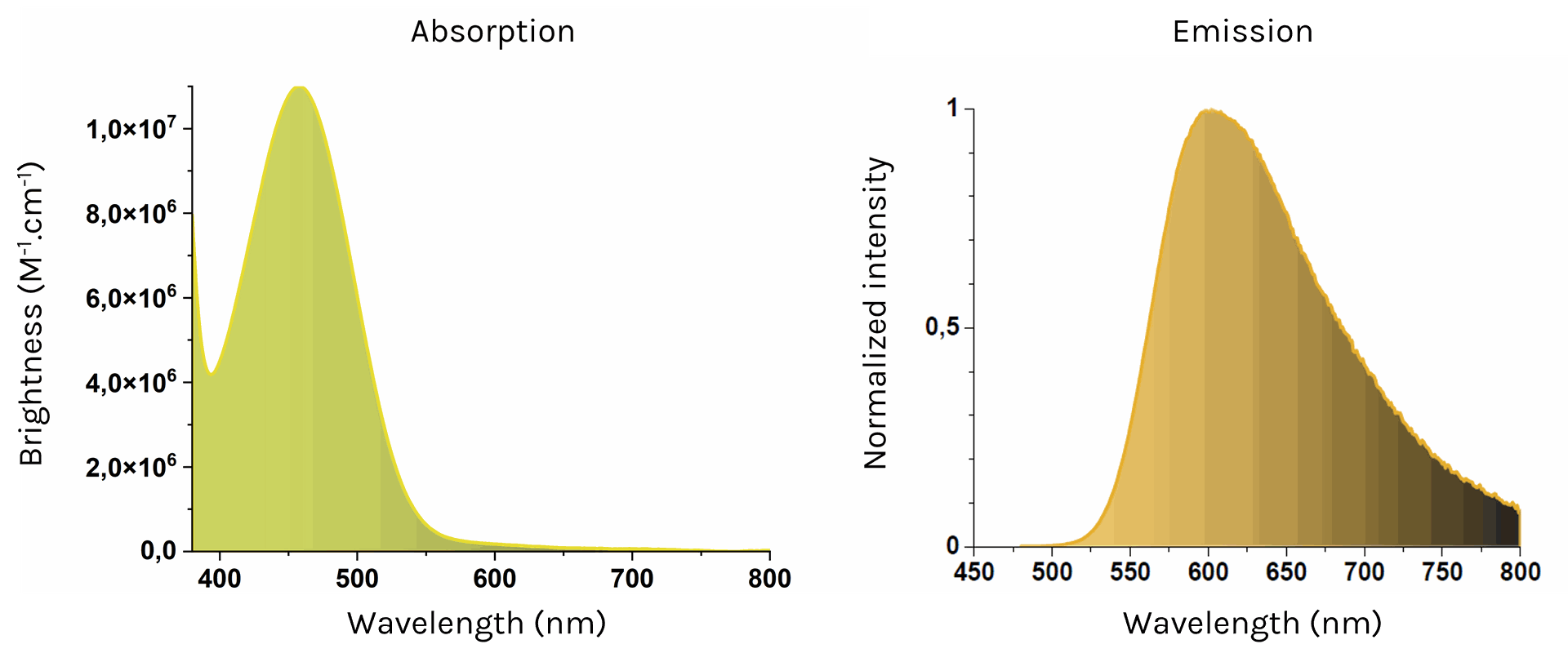
NanoTracer - Rheo kit
Absorption (left) and emission (right) spectra of NanoTracer - Rheo kit in water
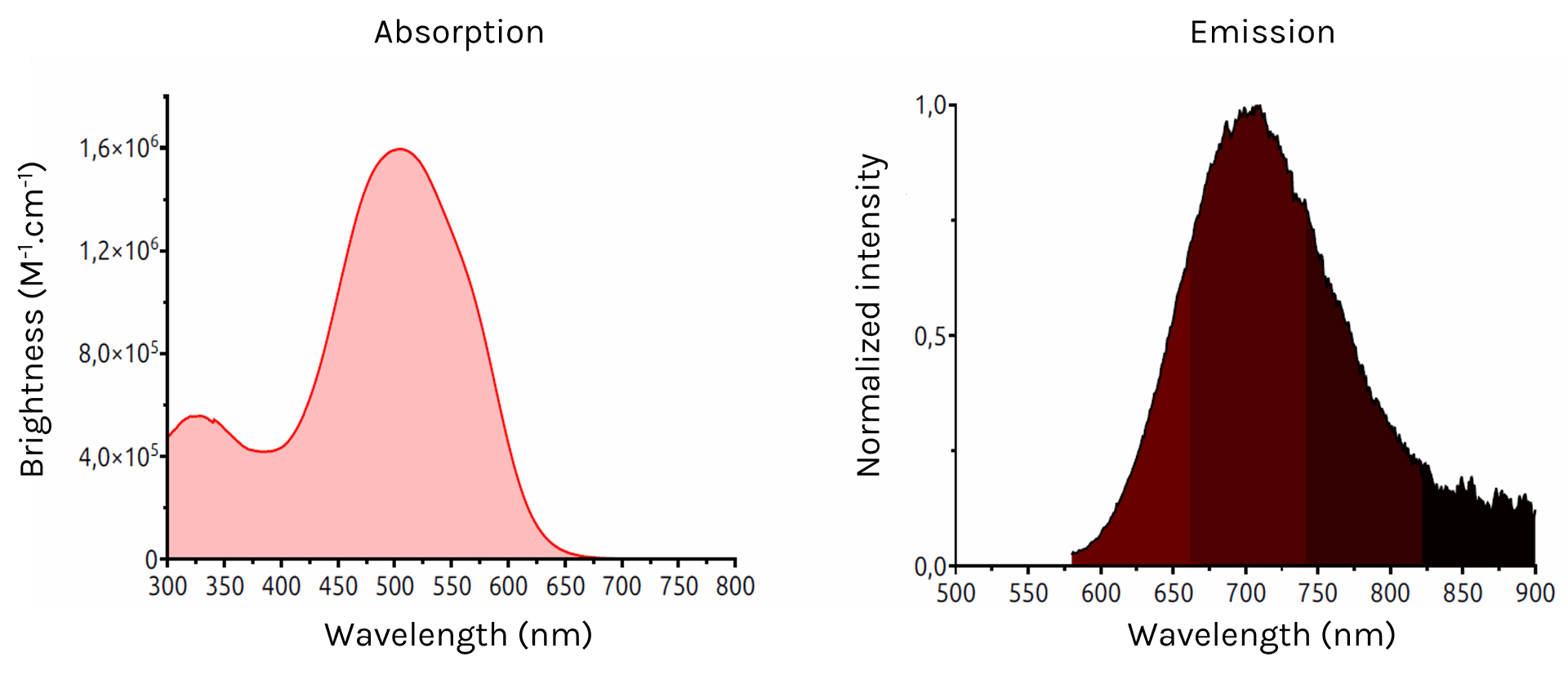
NanoTracer - Cell kit
Absorption (left) and emission (right) spectra of NanoTracer - Cell kit in water
Storage: NanoTracers can be stored for one year at 4°C, protected from light. Do not freeze.
Optimal dilution varies depending on the targeted application. Stock solutions should be diluted between 1:100 and 1:10000 for extracellular single-particle tracking experiments, or between 1:10 and 1:100 for intracellular single-particle tracking experiments.
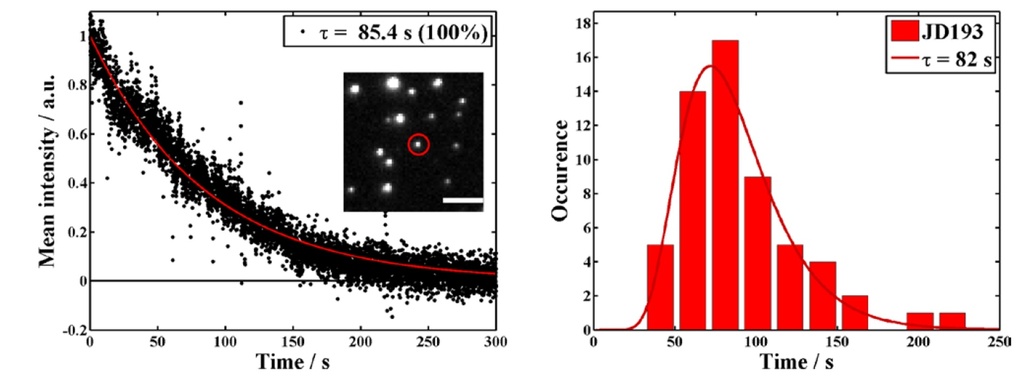
Single-molecule photostability study of NanoTracers shows longtime single-particle tracking capacity
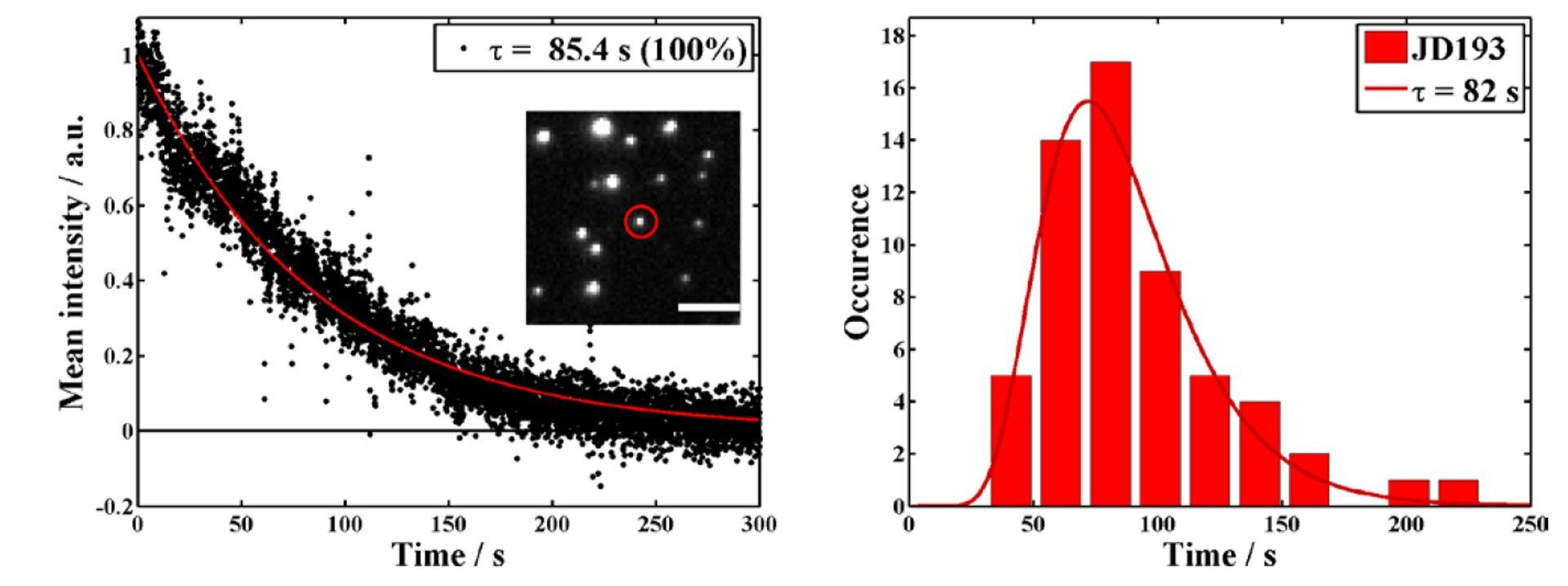
Source publication: Daniel J. et al, 2016
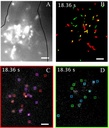
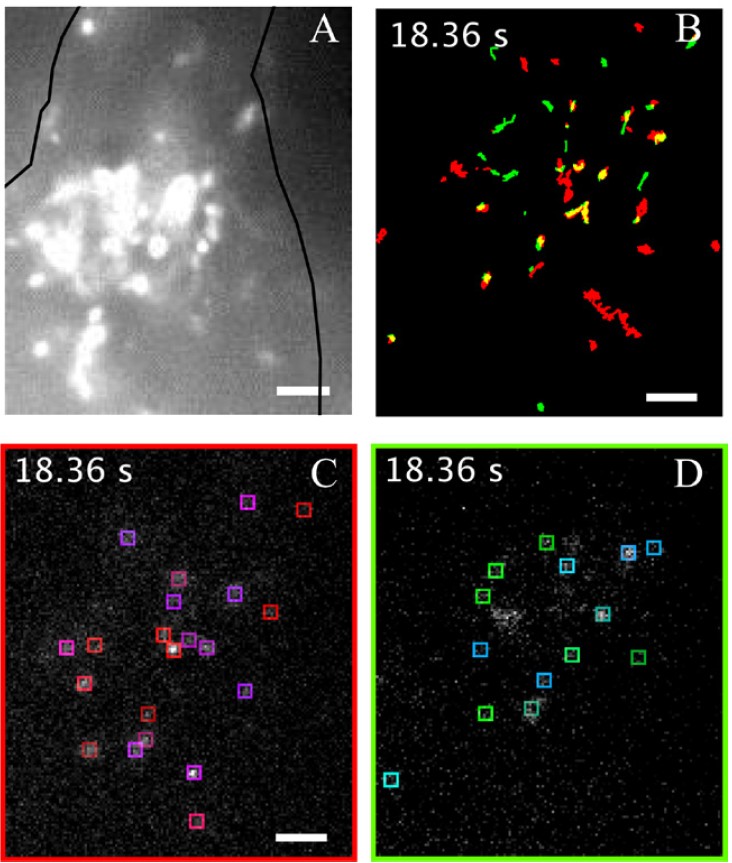
Single particle tracking of NanoTracers - Cell kit in live cells
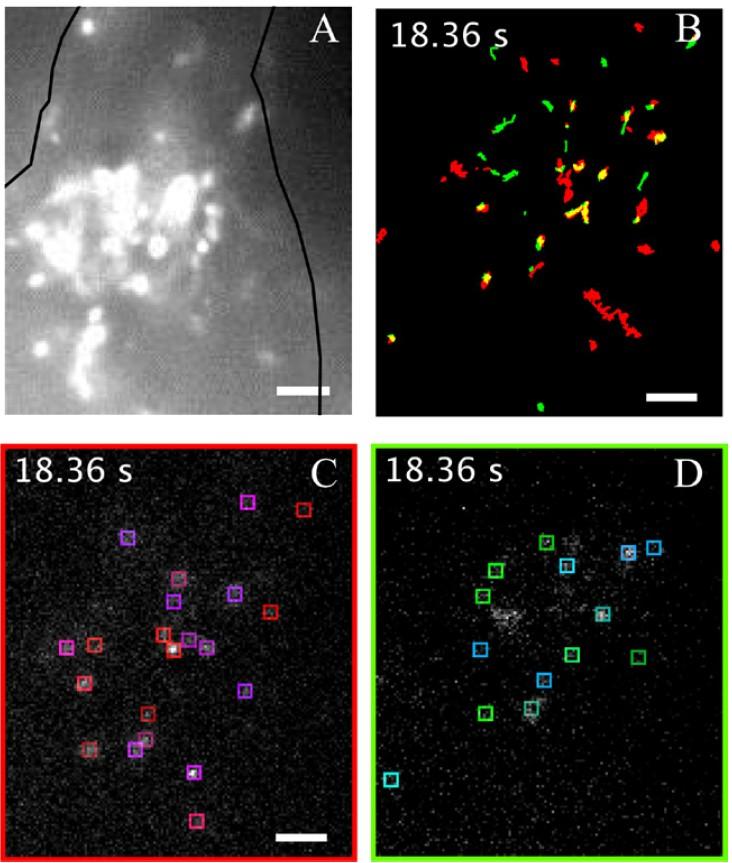
Source publication: Daniel J. et al, 2016
Interplay between NanoTracers - Rheo kit and live cells
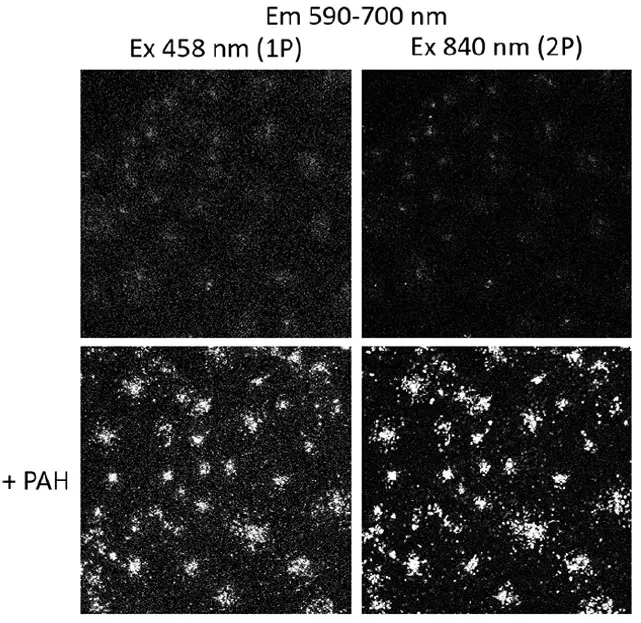
Representative 1P (left) and 2P (right) fluorescence images of bare (top) and PAH-coated (bottom) NanoTracers – Rheo kit incubated for 24h with Cos7 cells (1% v/v in cell culture medium) are provided. Maximal projections of z-stack images are displayed. Excitation wavelengths and emission detection ranges are indicated for each condition.
Source publication: Pagano P. et al, 2021
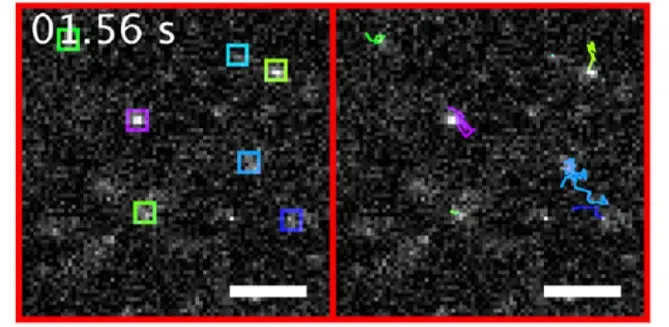
Single-particle tracking of NanoTracers in water
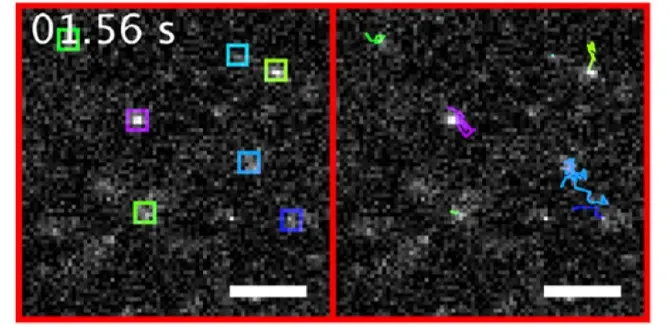
NanoTracers – Cell kit were imaged freely diffusing in water. The cumulative trajectories of the nanoparticles detected in each frame (left, in colored boxes) are shown on the right sub-panel using a corresponding color code. For each single nanoparticle tracked for more than four frames, its diffusion coefficient was extracted from its square displacements. Scale bars: 5 μm. A 488 nm laser power of 306 W·cm−2 was used for these experiments.
Source publication: Daniel et al, 2016
NanoTracer - Rheo kit:
Paolo Pagano, Morgane Rosendale, Jonathan Daniel, Jean-Baptiste Verlhac, Mireille Blanchard-Desce. Ultrabright Red to NIR Emitting Fluorescent Organic Nanoparticles Made from Quadrupolar Dyes with Giant Two-Photon Absorption (2PA) in the NIR Region. Confinement Effect on Fluorescence
and 2PA and Tuning of Surface Properties. Journal of Physical Chemistry C, 2021, 125 (46), pp.25695-25705. 10.1021/acs.jpcc.1c07831. hal-03457942
Rosendale M, Flores J, Paviolo C, Pagano P, Daniel J, Ferreira J, Verlhac JB, Groc L, Cognet L, Blanchard-Desce M. A Bottom-Up Approach to Red-Emitting Molecular-Based Nanoparticles with Natural Stealth Properties and their Use for Single-Particle Tracking Deep in Brain Tissue. Adv Mater. 2021 Jun;33(22):e2006644. doi: 10.1002/adma.202006644. Epub 2021 Apr 22. PMID: 33890332.
NanoTracer - Cell kit:
Jonathan Daniel, Antoine G Godin, Matthieu Palayret, Brahim Lounis, Laurent Cognet and Mireille Blanchard-Desce. Innovative molecular-based fluorescent nanoparticles for multicolor single particle tracking in cells. Journal of Physics D: Applied Physics, 2016, 49, pp.84002 - 84002. 10.1088/0022-3727/49/8/084002. hal-01390058
J. Daniel, A. G. Godin, G. Clermont, B. Lounis, L. Cognet, M. Blanchard-Desce, "NIR-emitting molecular-based nanoparticles as new two-photon absorbing nanotools for single particle tracking," Proc. SPIE 9523, International Conference on Nano-Bio Sensing, Imaging, and Spectroscopy 2015, 95230M (8 July 2015); https://doi.org/10.1117/12.2189638
Find below the protocol of use of our Everspark buffers:
- Everspark 1.0 Protocol
- Everspark 2.0 Protocol
- a video protocol for Everspark use with depression slides: How to mount a depression slide with Everspark
- a video protocol for Everspark use with glass bottom dishes: Everspark 2min protocol
- a video protocol for Everspark use with a chambered coverslip: Everspark use with chambered coverslips
Everspark technology has been intially developed by Karine Monier, Arnaud Favier and Christophe Place and and published in Scientific Reports:
Provost, A., Rousset, C., Bourdon, L. et al. Innovative particle standards and long-lived imaging for 2D and 3D dSTORM. Sci Rep 9, 17967 (2019). https://doi.org/10.1038/s41598-019-53528-0
Publications:
Nanoscale engagement and clusterization of Programmed death ligand 1 (PD-L1) in the membrane lipid rafts of Non-Small Cell Lung Cancer cells
Martina Ruglioni, Simone Civita, Tiziano Salvadori, Sofia Cristiani, Vittoria Carnicelli, Serena Barachini, Iacopo Petrini, Irene Nepita, Marco Castello, Alberto Diaspro, Paolo Bianchini, Barbara Storti, Ranieri Bizzarri, Stefano Fogli and Romano Danesi bioRxiv 2022.08.09.503318; doi: https://doi.org/10.1101/2022.08.09.503318
HIV-1 diverts actin debranching mechanisms for particle assembly and release in CD4 T lymphocytes
Rayane Dibsy, Erwan Bremaud, Johnson Mak, Cyril Favard, Delphine Muriaux
BioRxiv, December 16, 2022. doi. 10.1101/2022.12.15.520580
Fluorescent Polymer-AS1411-Aptamer Probe for dSTORM Super-Resolution Imaging of Endogenous Nucleolin
Fabre L, Rousset C, Monier K, Da Cruz-Boisson F, Bouvet P, Charreyre MT, Delair T, Fleury E, Favier A. Biomacromolecules. 2022 May 12. doi: 10.1021/acs.biomac.1c01706. PMID: 35549176
Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines.
Michalik S, Siegerist F, Palankar R, Franzke K, Schindler M, Reder A, Seifert U, Cammann C, Wesche J, Steil L, Hentschker C, Gesell-Salazar M, Reisinger E, Beer M, Endlich N, Greinacher A, Völker U. Haematologica. 2022 Apr 1;107(4):947-957. doi: 10.3324/haematol.2021.280154. PMID: 35045692
Metabolic biorthogonal labeling and dSTORM imaging of peptidoglycan synthesis in Streptococcus pneumoniae
Jennyfer Trouve, Oleksandr Glushonkov and Cecile Morlot
Star Protocols, December 13, 2021. doi: 10.1016/j.xpro.2021.101006
Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia.
Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U, Hentschker C, Michalik S, Steil L, Reder A, Schönborn L, Beer M, Franzke K, Büttner A, Fehse B, Stavrou EX, Rangaswamy C, Mailer RK, Englert H, Frye M, Thiele T, Kochanek S, Krutzke L, Siegerist F, Endlich N, Warkentin TE, Renné T.
Blood. 2021 Dec 2;138(22):2256-2268. doi: 10.1182/blood.2021013231. PMID: 34587242
Superresolution Microscopy of Drosophila Indirect Flight Muscle Sarcomeres.
Szikora S, Novák T, Gajdos T, Erdélyi M, Mihály J. Bio Protoc. 2020 Jun 20;10(12):e3654. doi: 10.21769/BioProtoc.3654. eCollection 2020 Jun 20. PMID: 33659324