- All Products
- UbiClear - clearing kit for biological tissues
UbiClear - clearing kit for biological tissues
https://www.idylle-labs.com/shop/ubiclear-clearing-kit-for-biological-tissues-531 https://www.idylle-labs.com/web/image/product.template/531/image_1920?unique=0dbe602Kit contents:
- 1x Depigmentation Mix
- 1x Clearing Base
- 1x Imaging Solution
A technology developed by Brigitte Delhomme & Martin Oheim (SPPIN - CNRS UMR 8003, Université Paris Cité)
UbiClear is an innovative method for optical clearing of biological samples. Thanks to its unique formulation, the UbiClear solution makes thick samples transparent in less than 20 minutes without any toxic compounds. Simply place your sample in the clearing buffer, incubate at 37°C and transfer it to the imaging solution. A great way to make clearing accessible to anyone!
UbiClear at a glance >
Ready to dive into deep tissue imaging ?
Non-toxic
Formulated without any harsh chemicals, UbiClear can be handled safely and does not deteriorate microscope objectives
Compatible with fluorescence labeling
UbiClear preserves endogenous fluorescence and can be combined with immunostaining protocols
Ultra-fast
Stop spending days on clearing: clear your samples within minutes thanks to a 1-step clearing method
Versatile
Clears a vast panel of soft & hard mammalian tissues (bone, teeth), insects & plants. Nothing can stop it!
How does UbiClear work?
UbiClear is an aqueous solution that modifies the optical properties of complex biological tissues by removing lipids and homogenizing refractive indexes. This renders opaque tissues transparent while keeping their original structure intact. As a result, light passes through specimens without any scattering or absorption, yielding high-quality images of entire 3D structures. The UbiClear workflow is straight-forward, and does not require any specific expertise or access to sophisticated equipment.
Depigmentation (1h)
Remove sample coloration by immersing it into UbiClear Depigmentation Buffer for 1h at 37°C.
Clearing (20min - overnight)
Incubate your sample into the UbiClear clearing solution at 37°C until fully transparent.
Maximal transparency is achieved in a few minutes for most tissue fragments (<20 min for mouse cerebral cortex and small intestine), and may take up to a few hours for entire organisms.
Imaging & storing
Transfer your cleared sample to the post-clearing solution (RI = 1.47) for imaging. Cleared samples can be safely stored in the post-clearing solution at 4°C for years.
Repeat !
If needed, samples can be re-opacified simply by transferring them to 0.2 M Tris buffer.
Discover more on Ubiclear & its applications by hearing directly from its inventors: http://brg.sppin.fr
UbiClear applications
Sample types: UbiClear can be used to clear a wide spectrum of samples ranging from small 3D cell structures up to entire organisms. So far, UbiClear has shown efficient clearing of:
- 3D cell structures: human embryoid bodies, human mini-brains (1 mm diameter).
- Human tissues: frontal cortex, artery segments (4 mm diameter x 5 mm length)
- Mice tissue sections (up to 1 mm thick): spinal cord, brain (cerebellum, choroid plexus), testes, seminal vesicle, ear (elastic cartilage and hair), lungs, heart, intestine, kidney, skeletal muscle, yolk sac membranes
- Ox tissues (up to 1 mm thick): knee (joint cartilage and bone)*
- Plants: ivy leaves, carrot slices, beet roots, microcallus & callus organoids, nasturtium stamen, melon seeds, sprouted tomato seeds, rose stem slices
- Whole organs: mouse brain, heart, intestine, eye, teeth*, dorsal root ganglia
- Whole organisms: Colisa Lalia, adult zebrafish, firebugs (Pyrrhocoris apterus), wasps, grasshoppers, shield bugs (Graphosoma lineatum), flies, cockroaches, spiders
- Crustaceans: woodlouse
- Molluscs: slug, mussel (including shell)*
*for these samples, an additional step of demineralization needs to be performed before clearing.
Compatible assays: label-free anatomical studies (autofluorescence, volumetric imaging), dye-based assays (Evans blue, albumin-FITC), immunostaining. UbiClear preserves endogenous fluorescence.
Microscopy techniques: confocal, spinning-disk, light-sheet microscopes*.
*Extra diluting buffer can be purchased separately to make more imaging solution if needed (i.e. for use with light sheet microscopes).
Additional resources
How to use Ubiclear in pictures
Download Protocol
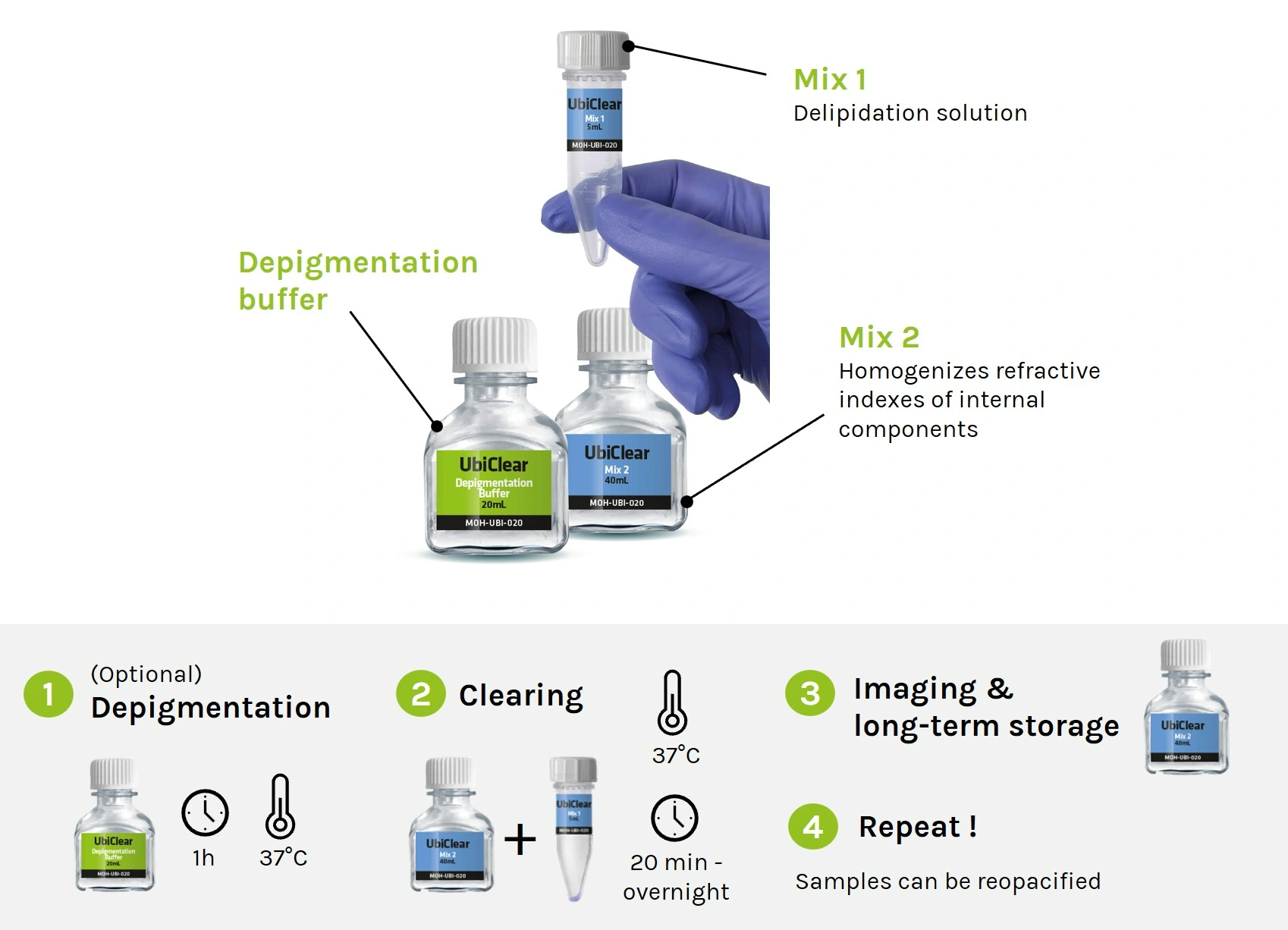
Storage: The depigmentation buffer, Mix 1 and Mix 2 solutions should be kept at room temperature, protected from light. In these conditions, all solutions can be stored for at least 1 year.
Kit contents of UbiClear:
Small kit
-
Depigmentation Buffer: 20 mL
Mix 1: 5 mL
Mix 2: 40 mL
Final volume of depigmentation, clearing & imaging solutions = 20 mL each*
Large kit
-
Depigmentation Buffer: 200 mL
Mix 1: 50 mL
Mix 2: 400 mL
Final volume of depigmentation, clearing & imaging solutions = 200 mL each*
*Mix 1 & Mix 2 are mixed at a 1:5 ratio to make the final clearing solution. Mix 2 will be added with water to make up the final imaging solution.
An extra 400 mL volume of Mix 2 can be purchased separately to make more imaging solution if needed (i.e. to fill out light sheet microscope tanks). A final volume of 400-500 mL of imaging solution can be prepared using the 400 mL of Mix 2 provided.
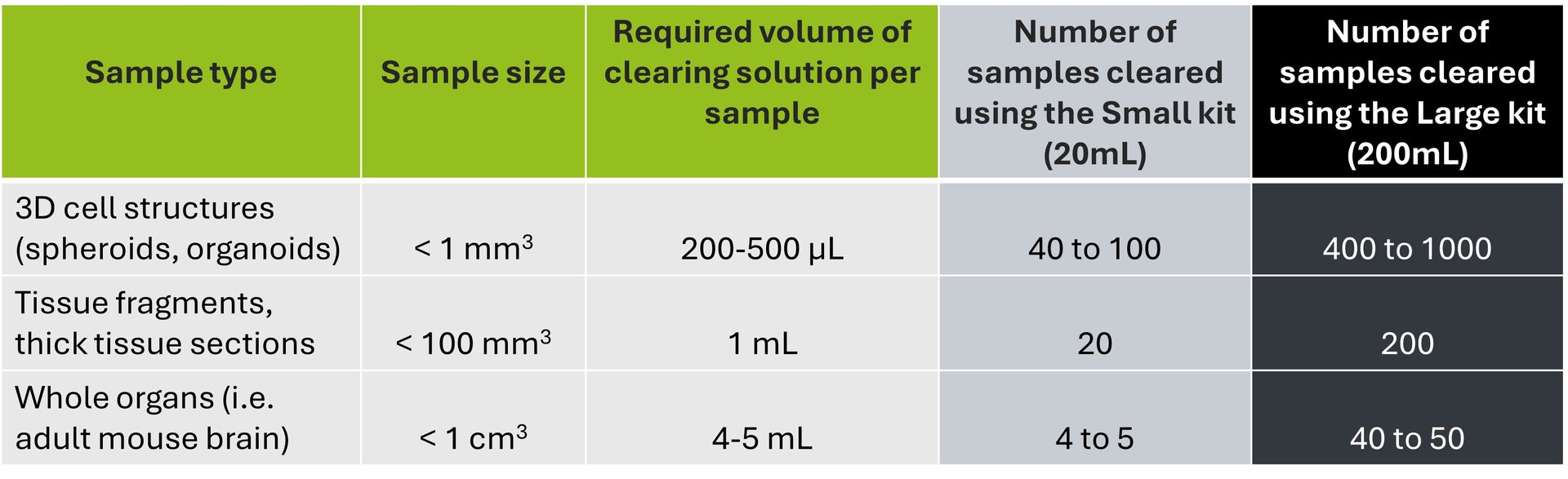
The volume of clearing solution required varies according to your sample size. As a general rule, we recommend using a clearing solution volume corresponding to 10X your sample volume. Please refer to the table below to find out which kit format will best suit your needs.
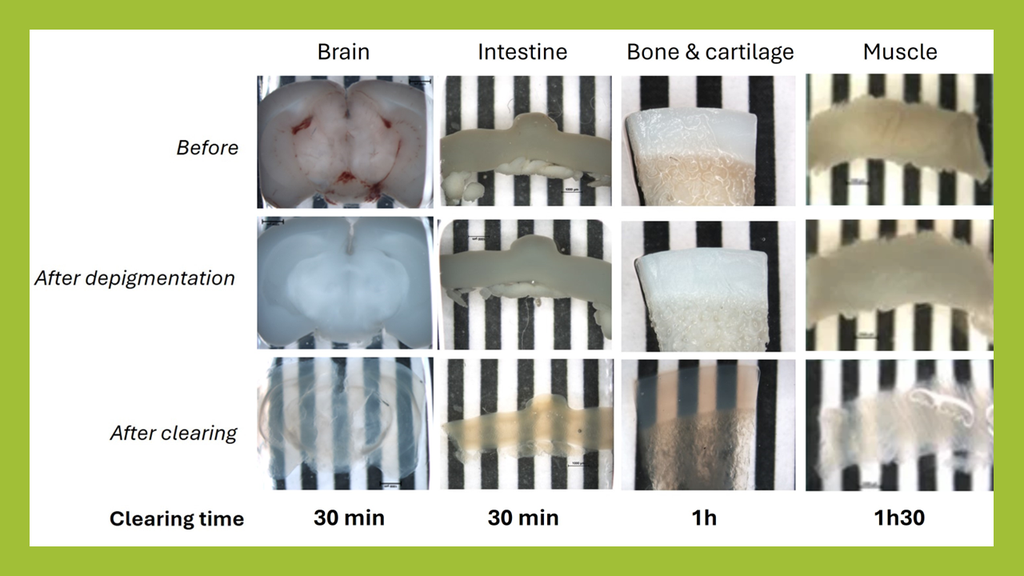
Fast & versatile clearing of diverse animal tissues using UbiClear
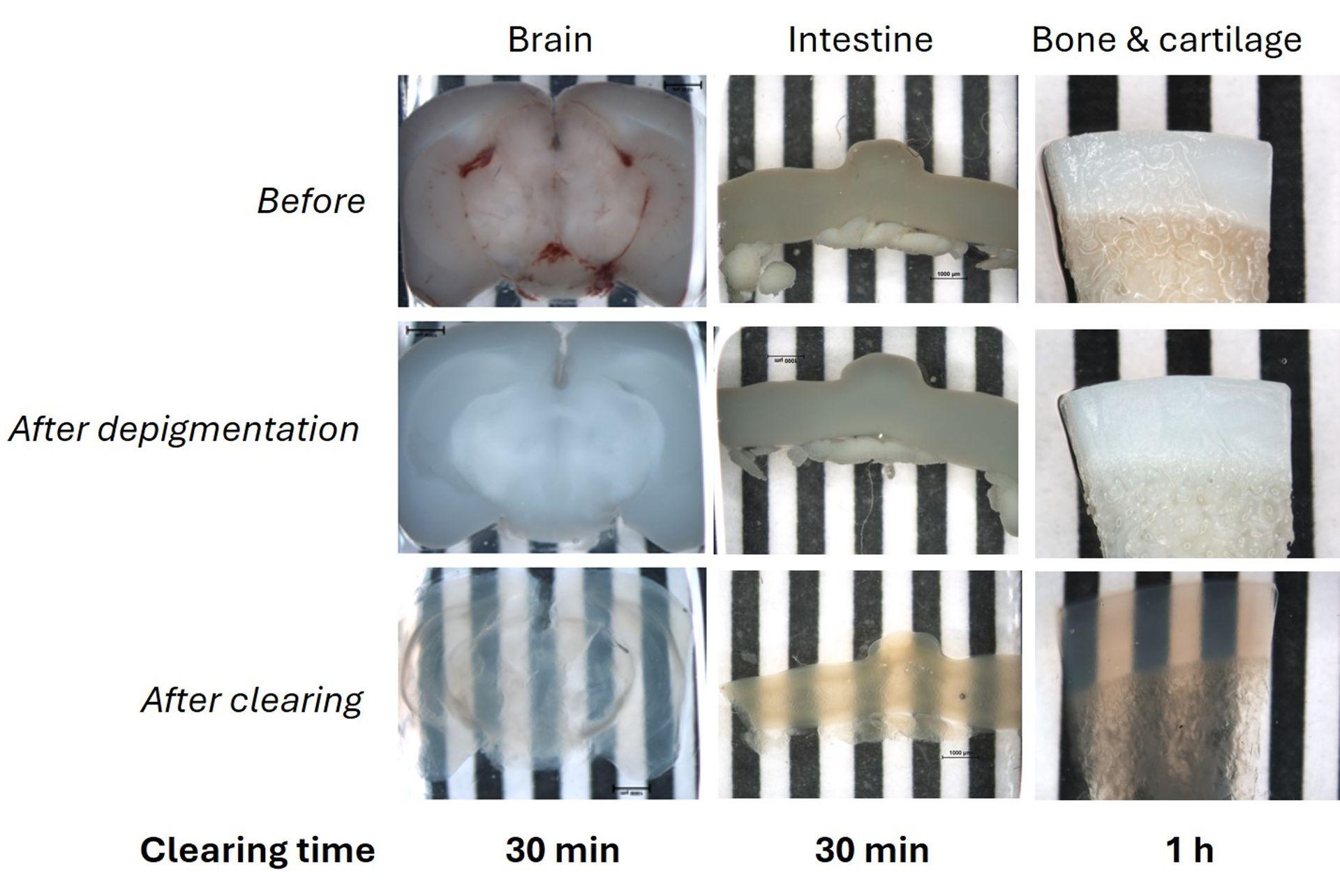
1 mm thick mice brain slices (fat tissue), 2 cm mice intestine fragments and a 1 mm thick ox knee slices (joint cartilage and bone, calcified tissue) were cleared using the UbiClear method and imaged on a striped target. Ox knee joint samples were decalcified and all samples depigmented and cleared using UbiClear. The target stripes are visible through the sample after clearing for less than an hour.
Credits: Brigitte Delhomme & Martin Oheim (CNRS, SPPIN UMR8003)
Label-free 3D morphological characterization of the deep enteric nervous system
Source publication: Hazart D. et al, 2023
Visualizing Glial fibrillary acidic protein (GFAP) expression in mouse brain
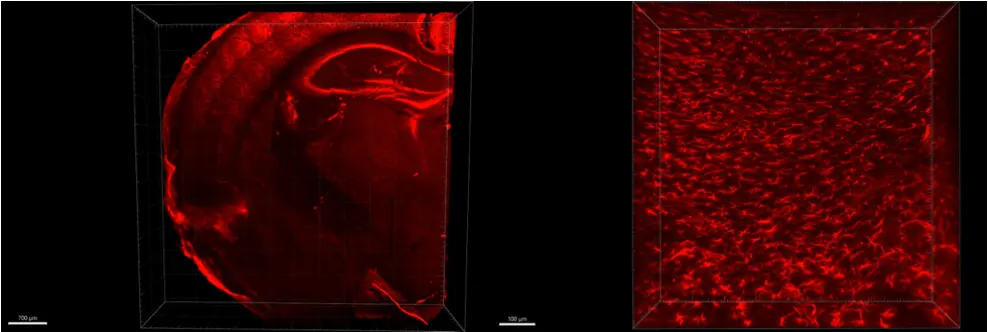
Indirect immunofluorescence and clearing was performed on 1 mm thick sections of formaldehyde-fixed mouse brain using GFAP antibody and secondary antibody Alexa 555 fluorophore. On the left the whole slice was imaged in mosaic with 10XNa0.3 objective, on the right 40XNa1.0 was imaged in the cortex of the mouse brain.
Credits: Diane Derrien, Brigitte Delhomme & Martin Oheim (CNRS, SPPIN UMR8003)
Cleared mouse dorsal root ganglia combined with immunohistochemistry
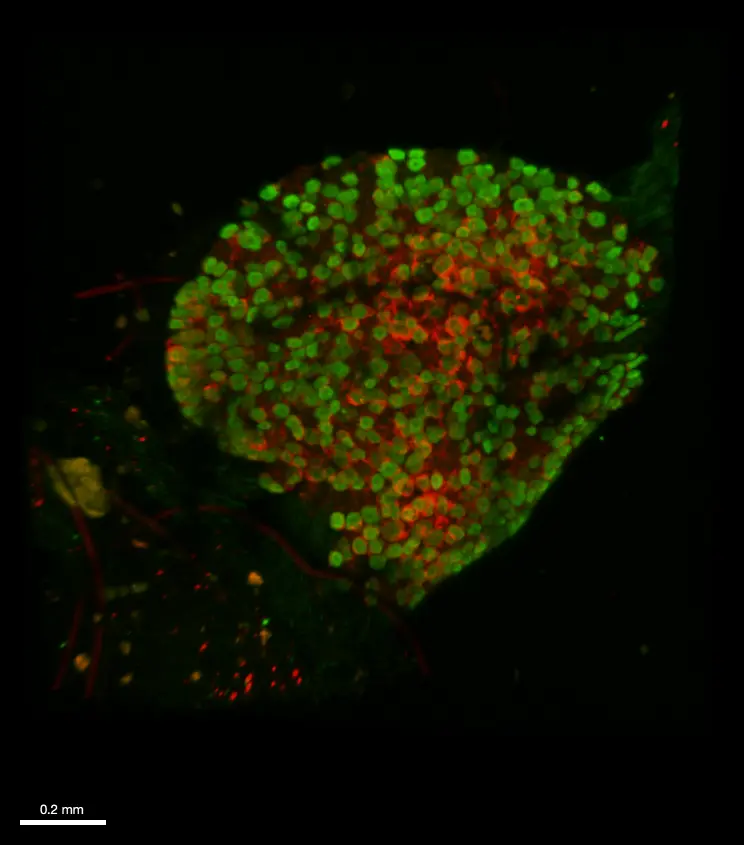
Immunofluorescence and clearing was performed on whole fixed dorsal root ganglia from a transgenic mouse model that expresses both (i) a genetically-encoded GCaMP6 calcium sensor in sensory neurons as well as (ii) a hematoglutinin-tagged chemogenetic receptor in satellite glial cells. An anti- GFP (green) and an anti-hematoglutinin (red) antibodies were used to amplify GCaMP6 staining and reveal satellite glial cells, respectively. The whole ganglia was imaged using a confocal microscope Axio LSM710 (Zeiss) and 10XNa0.3 WD 5mm air objective, in 620 µm depth and z 2 µm. 3D reconstruction was obtained with Imaris viewver software (oxford instruments).
Credits: Andreia Ramos (Université Paris Cité, CNRS, INCC UMR8002, Cendra Agulhon’s Group) and , Brigitte .Delhomme (Université Paris Cité, CNRS, SPPIN UMR8003, Martin Oheim’s laboratory). Courtesy of Cendra Agulhon.
Fast clearing of human calcified arteries with UbiClear
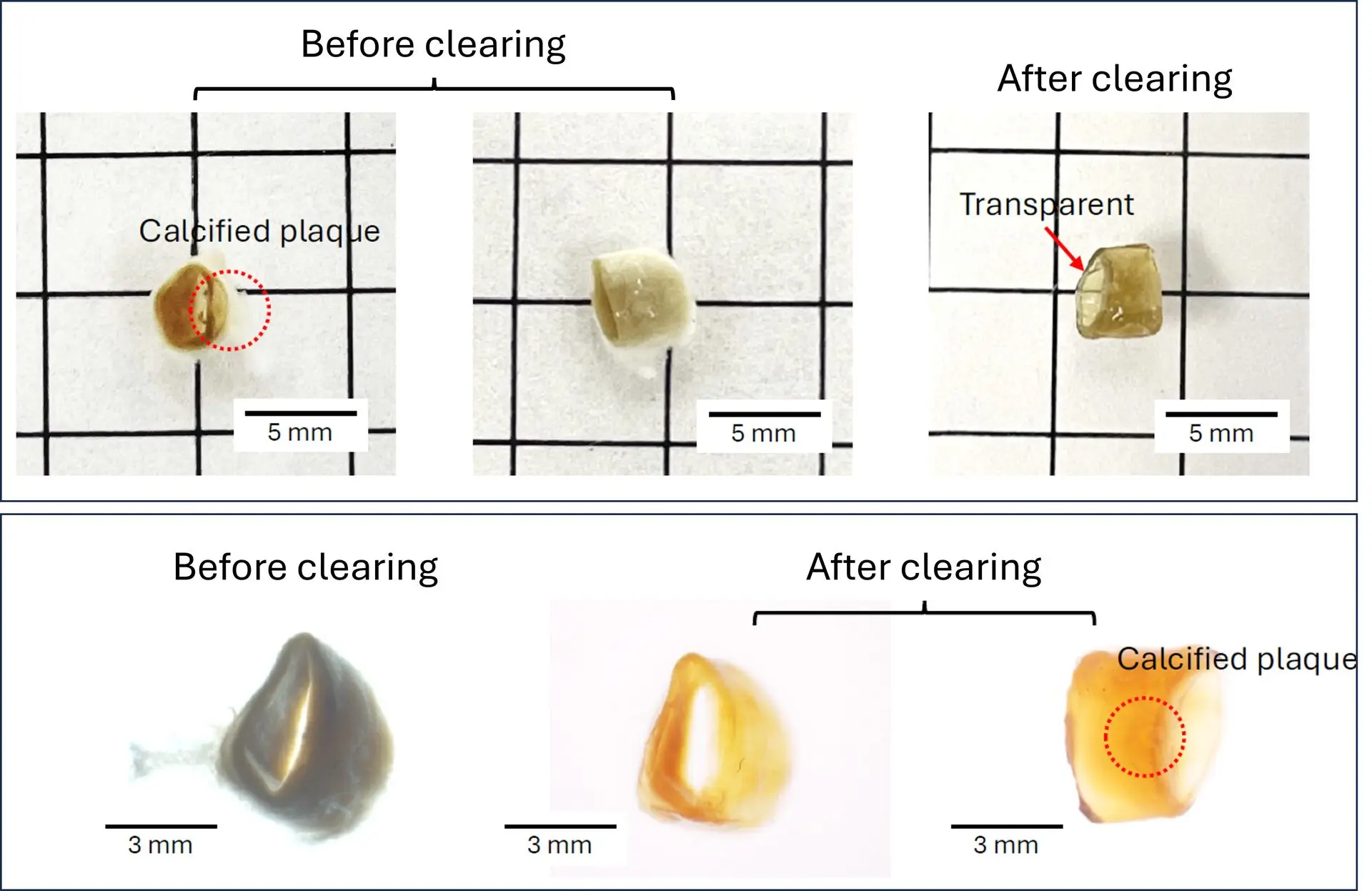
A human calcified artery (4 mm diameter x 5 mm length) was fixed with 4% PFA and incubated in the Ubiclear solution for 2h at 37°C. Phase-contrast images show optimal transparency without the need to perform the optional depigmentation & demineralization steps.
Credits: Dr. Linxia Gu & Yingnan Zhai, Florida Institute of Technology – Biomedical Engineering and Science Department, US - 2024
Mapping Iba1 expression in a cleared mouse spinal cord
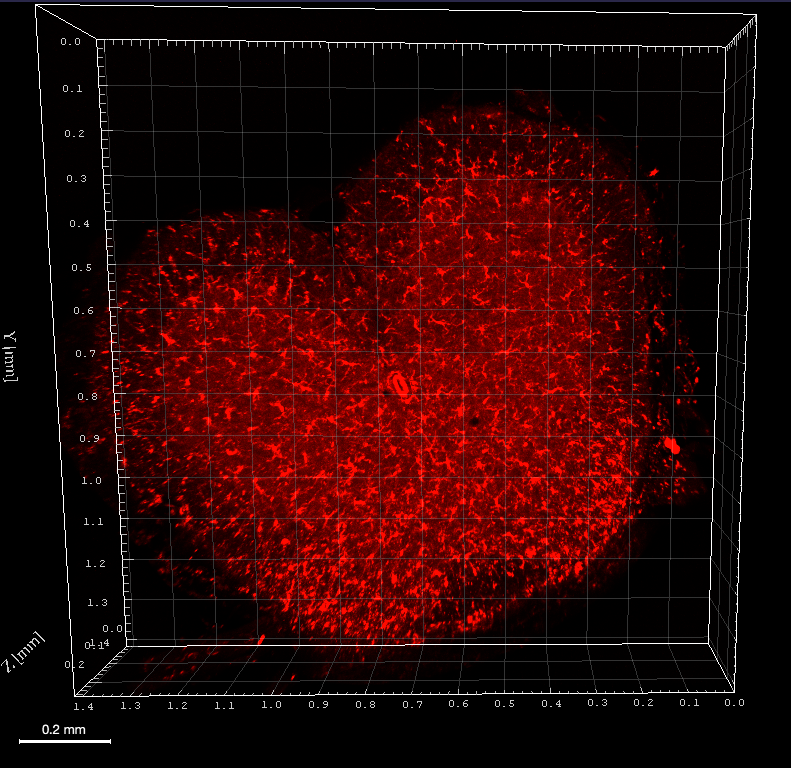
Immunofluorescence was performed on a 500µm mouse spinal cord section after depigmentation. Iba1 antibody, a rabbit polyclonal antibody, and Cy3 secondary antibody were used for incubations. Iba1 was imaged using an Axio LSM710 (Zeiss) confocal microscope with 10XNa0.3 WD 5mm air objective, zoom 0.6 in 270µm depth and z 2µm. 3D reconstruction was performed using Imaris viewver software (Oxford Instruments).
Credit : Clearing B.Delhomme & M.Oheim (CNRS, SPPIN UMR8003)
Insect clearing using UbiClear
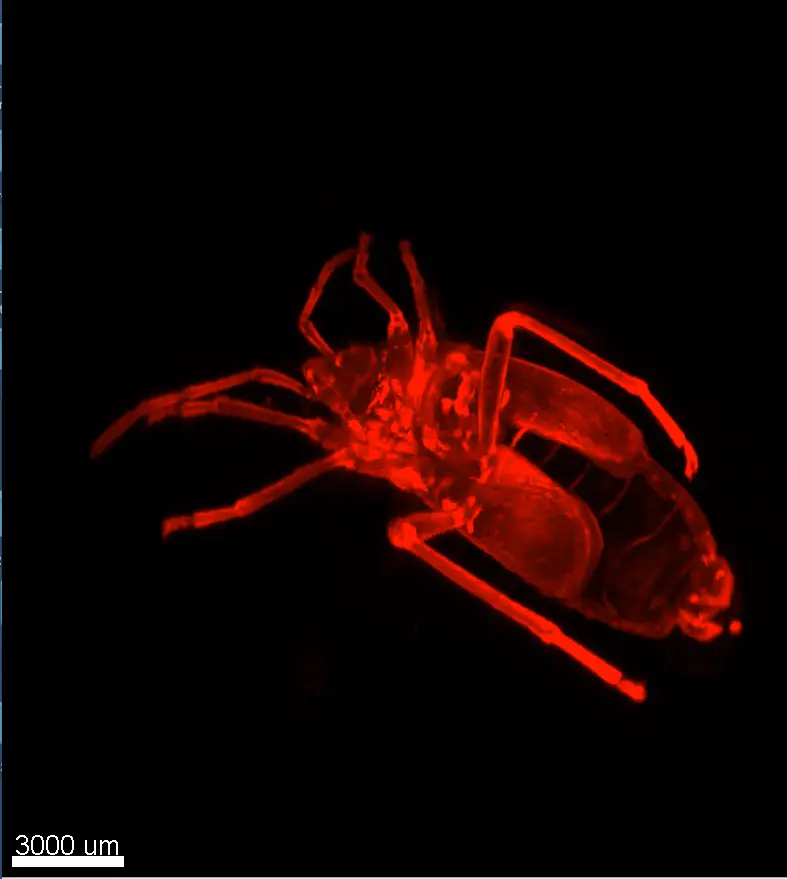
Credits: Clearing: Brigitte Delhomme (CNRS, SPPIN UMR 8003), Imaging: J. Fernandes (Institut Pasteur, Paris) & Martin Oheim (CNRS, SPPIN UMR8003)
Whole brain clearing using UbiClear
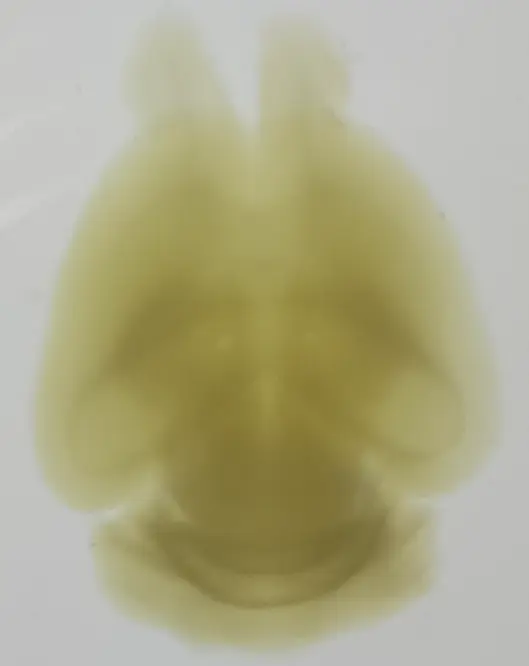
A whole brain from a 4-month old mouse was depigmented and cleared using UbiClear. The picture was taken 48h after clearing.
Credits: Brigitte Delhomme & Marwa Moulzir (CNRS, SPPIN UMR 8003)
One-step clearing of the small intestine
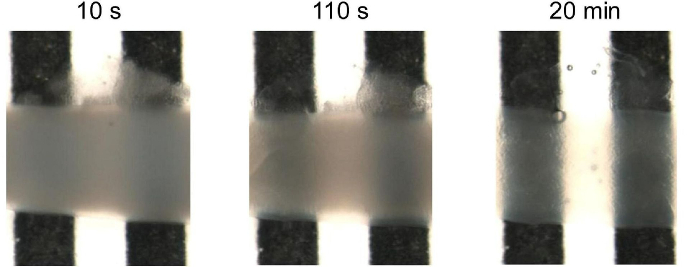 A 1.5 cm long mice intestinal fragment was cleared using UbiClear and placed in a home-made tank with a black-and-white stripe pattern. Images were acquired on a SMZ800 stereo macroscope (Nikon, Amstelveen, The Netherlands) after 10sec, 110sec and 20min of incubation in the UbiClear clearing solution. Michelson’s contrast calculation revealed that 71% transparency was reached in less than 15 min.
A 1.5 cm long mice intestinal fragment was cleared using UbiClear and placed in a home-made tank with a black-and-white stripe pattern. Images were acquired on a SMZ800 stereo macroscope (Nikon, Amstelveen, The Netherlands) after 10sec, 110sec and 20min of incubation in the UbiClear clearing solution. Michelson’s contrast calculation revealed that 71% transparency was reached in less than 15 min. Source publication: Hazart D. et al, 2023
Imaging deep blood vessel network in mouse cerebellum
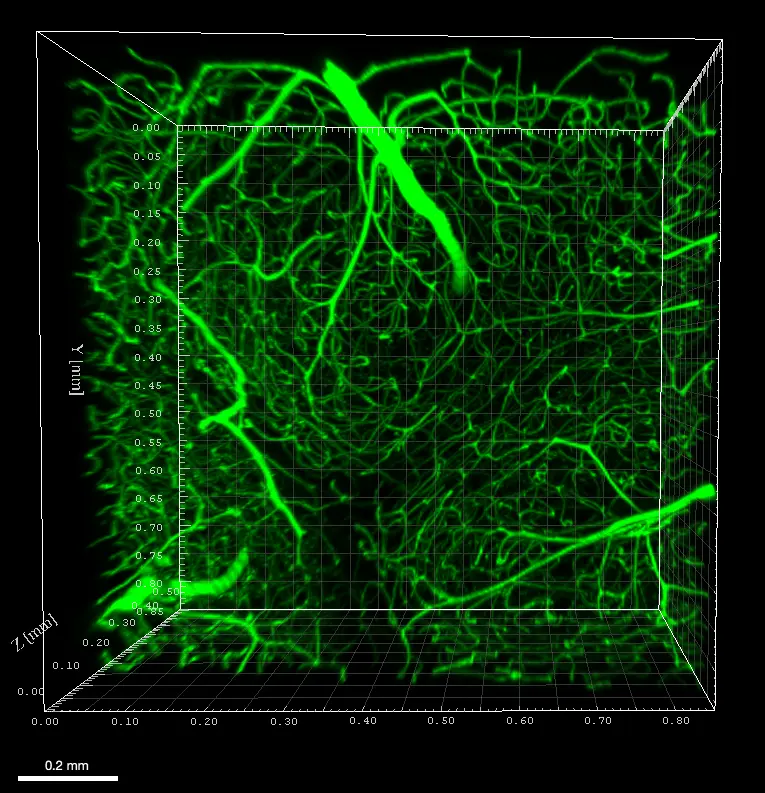 Mouse was perfused with FITC albumin and sacrificed to image blood vessels in the cerebellum. A 500µm tissue slice was cleared without depigmentation and imaged using an Axio LSM710 (Zeiss) confocal microscope with 488nm laser and 10XNa0.3 WD 5mm air objective, in 508µm depth and z 4µm. 3D reconstruction was performed using Imaris viewer software (Oxford Instruments).
Mouse was perfused with FITC albumin and sacrificed to image blood vessels in the cerebellum. A 500µm tissue slice was cleared without depigmentation and imaged using an Axio LSM710 (Zeiss) confocal microscope with 488nm laser and 10XNa0.3 WD 5mm air objective, in 508µm depth and z 4µm. 3D reconstruction was performed using Imaris viewer software (Oxford Instruments).Credits : Mouse painting vessels X.Bai (University of Saarland, Homburg (DE)), Clearing B.Delhomme (CNRS, SPPIN UMR8003, Paris (Fr)) , F.Licata (UMS Paris-cité University (Fr) & Martin Oheim (CNRS, SPPIN UMR8003, Paris (Fr))
Detailed morphological characterization of the enteric nervous system
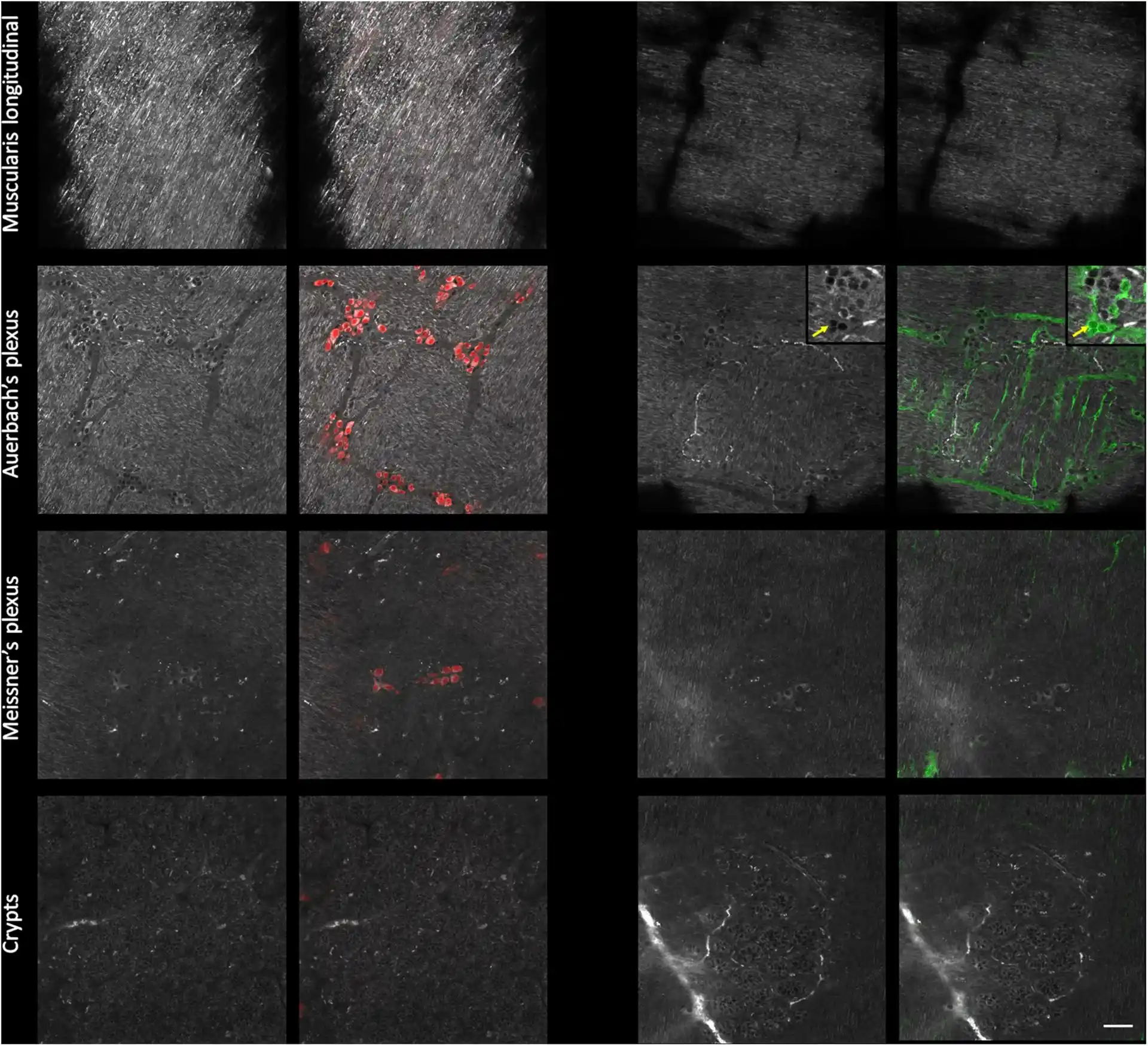 Mice small intestine fragments were cleared using UbiClear and immunolabeled against a pan-neuronal marker (red) and an enteric-glia marker (green). Single autofluorescent images (grayscale) and fluorescence images were taken at different depths in the intestinal wall (muscularis longitudinalis, Auerbach’s plexus, Meissner’s plexus and crypts). Images were acquired on a LSM880 inverted confocal Airyscan microscope (Zeiss).
Mice small intestine fragments were cleared using UbiClear and immunolabeled against a pan-neuronal marker (red) and an enteric-glia marker (green). Single autofluorescent images (grayscale) and fluorescence images were taken at different depths in the intestinal wall (muscularis longitudinalis, Auerbach’s plexus, Meissner’s plexus and crypts). Images were acquired on a LSM880 inverted confocal Airyscan microscope (Zeiss). Source publication: Hazart D. et al, 2023
Publications using UbiClear:
Hugo Touja, Frédéric Brau, Thierry Bastien, Brigitte Delhomme, Martin Oheim (2024) M-Cube — towards correlative multi-scale light-sheet imaging using a compact, modular and moving illuminator. EPJ Web Conf. 309 04013. doi: 10.1051/epjconf/202430904013
Hazart D, Delhomme B, Oheim M and Ricard C (2023) Label-free, fast, 2-photon volume imaging of the organization of neurons and glia in the enteric nervous system. Front. Neuroanat. 16:1070062. doi: 10.3389/fnana.2022.1070062.
Doriane Hazart, Malvyne Rolli-Derkinderen, Brigitte Delhomme, Pascal Derkinderen, Martin Oheim and Clément Ricard (2024) L’intestin, lanceur d’alerte, dans les prémices de la maladie de Parkinson. Med Sci (Paris), 40 6-7 (2024) 544-549. doi: https://doi.org/10.1051/medsci/2024082
Ricard, C., D. Hazart, B. Delhomme, M. Moulzir, I. Rakotoson, and M. Oheim. 2025. “ Fast Tissue Clearing and Volume Imaging Techniques for Anatomy.” Microscopy Research and Technique 1–14. https://doi.org/10.1002/jemt.70013.
Discover all posters featuring UbiClear at the AFH (French Association of Histotechnology) congress:
UbiClear relies on an aqueous clearing solution, formulated without any harmful solvents or expensive components, allowing to make thick biological samples transparent in no time. By providing a safe and affordable technique even when large volumes are needed, UbiClear was designed to democratize tissue clearing for researchers interested in deep tissue imaging.
UbiClear can be used to clear a wide spectrum of samples ranging from small 3D cell structures up to entire organisms. So far, UbiClear has shown efficient clearing of:
- 3D cell structures: human embryoid bodies, human mini-brains (1 mm diameter).
- Human tissues: frontal cortex.
- Mice tissue sections (1 mm thick): spinal cord, brain (cerebellum, choroid plexus), testes, seminal vesicle, ear (elastic cartilage and hair), lungs, heart, intestine, kidney, skeletal muscle, yolk sac membranes.
- Ox tissues (1 mm thick): knee (joint cartilage and bone)*.
- Plants: ivy leaves, carrot slices, beet roots, microcallus & callus organoids, nasturtium stamen, melon seeds, sprouted tomato seeds, rose stem slices
- Whole organs: mouse brain, heart, intestine, eye, teeth* and dorsal root ganglia.
- Whole organisms: Colisa Lalia, zebrafish, firebug (Pyrrhocoris apterus), wasp, grasshopper, spiders, shield bugs (Graphosoma lineatum), flies, cockroaches.
- Crustaceans: woodlouse.
- Molluscs: slug, mussel (including shell)*
*for bone & teeth samples, an additional step of demineralization needs to be performed prior clearing.
We would greatly appreciate your valuable inputs by helping us complementing this list with any of your positive research experiences. Drop us a message through our Contact page!
Yes, it is possible to re-opacify the samples by incubating them in a 0.2 M Tris buffer at pH 8. Re-opacifying can be a practical way to select and section a region of interest for downstream assays (i.e. immunostaining).
The final imaging solution obtained following our protocol of use (i.e. diluting the Mix 2 solution in water) has a refractive index of 1.47. It is possible to increase it up to 1.485 by adding less water to the Mix 2 solution.
UbiClear efficiently clears biological tissues by removing lipids (Mix 1) and homogenizing refractive indexes (Mix 2) of the sample's internal components. The clearing solution is prepared by mixing Mix 1 & Mix 2, and the imaging solution is prepared by diluting Mix 2 in water.
Yes, endogenous fluorescence is preserved upon clearing (this has been shown with dT-tomato and GFP). If your sample contains a fluorescent native protein, you simply need to skip the optional depigmentation step as this will likely interfere with the fluorescence signal.
Yes, it is possible to perform immunostaining and clear your labeled sample with UbiClear afterwards.
Immunostaining using AF405, AF488, AF555, AF647, Cy3, TMR, AM-cyan &Td tomato have been successfully performed on samples cleared with UbiClear.
Yes, UbiClear can be combined with direct dyes for visualizing cellular or structural features. This has been validated with Evans blue and albumin-FITC for imaging blood vessels in cleared samples. UbiClear is not compatible with common direct nucleic acid dyes (DAPI, BET, methyl green, Topro3…). If DNA staining is expected, we recommend using indirect antibody labeling strategies.
So far, the UbiClear kit has been successfully used on samples cleared using 10% formalin (or 4% PFA), and that is therefore the fixation method we recommend. It could be that clearing works well with other fixation solutions, yet we can't guarantee that clearing efficiency will be similar.
Once cleared, samples can be imaged using conventional confocal microscopes (for samples up to ~500 µm thick). Thicker samples may be imaged using light-sheet microscopes.
Need some advice? We would be happy to answer any questions on the choice of microscopy technique, data analysis and handling. Feel free to reach out at [email protected].
Clearing time will vary depending on your sample thickness and internal properties. Past experiments demonstrated that 30 minutes were sufficient to clear 1 mm-thick samples (i.e. brain organoids, mice intestine fragments), for a total procedure not exceeding 2 hours. The protocol might be extended up to 72 hours for entire organisms.
As UbiClear works by removing lipids from the samples, this solution will not be suitable for imaging & staining lipids. It is also not compatible with direct nucleic acid dyes (DAPI, BET, methyl green & Topro3). If DNA staining is expected, we recommend using indirect antibody labeling strategies.
Sample shrinkage has been observed within the first few minutes of incubation in the clearing solution, which is explained by the difference in osmolarity between the sample and the solution leading to dehydration. This effect is then attenuated after a few minutes as the clearing solution penetrates inside the structures.
No, UbiClear does not contain any organic solvents neither volatile compounds. Its handling does not require the use of a chemical hood or sophisticated ventilation systems for imaging.
The UbiClear kits include a Depigmentation Mix, a Clearing Base and some Imaging Solution. Everything you need to have on your end is some water, 0.2M Tris at pH 8 and some 30% hydrogen peroxyde.
The volume of clearing solution required varies according to your sample size. As a general rule, we recommend using a clearing solution volume corresponding to 10X your sample volume. Please refer to the table below to find out which kit format will best suit your needs.
Yes, our imaging solution does contains an anti-fading agent used to preserve fluorescence of tissues and cell structures. The Ubiclear imaging solution has been notably used for confocal, light-sheet and 2-photon imaging without any noticeable fading effects. A potential risk of fluorescence decay may arise if the solution is used without adding any water to it (although we recommend adding water at a 1:5 ratio to achieve a refractive index of 1.47, the solution may be used as it is when a higher refractive index is needed).
Additional Mix 2 is usually needed when using microscopes that include a large tank or chamber to be filled with imaging solution (this is typically the case with light-sheet microscopes, or more generally setups where whole organisms or large samples need to be imaged over extended periods). If this is your case, we would recommend buying an additional bottle of Mix 2 to make your required amount of imaging solution. If not, the final volume of imaging solution needed will be similar to that of the clearing solution, so the amount of Mix 2 included in the kit is sufficient.
We recommend using the imaging solution provided in the kit (Mix 2) to ensure that the sample is imaged in a solution of a similar composition to that of the clearing solution used beforehand, and therefore minimize the potential artifacts linked to imaging solution heterogeneity. Indeed, the imaging solution prepared as per the Ubiclear protocol will have the exact same composition as the clearing solution without the detergents to preserve the sample integrity and microscope objectives. Moreover, the Ubiclear solution has been specifically formulated to reduce photobleaching, as well as to re-equilibrate refraction indexes and reverse any potential shrinking that might have happened upon clearing.
Especially, we do not recommend using oils as imaging solutions due to the high water content and poor fat solubility of the clearing solution.
Recent assays showed that Ubiclear efficiently cleared gliolastoma in thick mice brain slices. The tumours might take longer to clear (probably due to the increased local compactness of the tissues). To give you an idea, the healthy brain tissue was transparent in an hour only while 18 hours were necessary to make the whole slice (including tumour regions) equally transparent.
Once cleared, samples can be kept in the post-clearing solution at 4°C for long-term storage (entire insects have been imaged 5 years after clearing!)
UbiClear can be used to clear a wide spectrum of samples ranging from small 3D cell structures up to entire organisms. So far, UbiClear has shown efficient clearing of:
- 3D cell structures: human embryoid bodies, human mini-brains
- Human tissues: frontal cortex
- Mice tissues: spinal cord, cerebellum, brain (1 mm sections), testis, seminal vesicle, ear (elastic cartilage and hair), lungs, heart, intestine, liver, kidney, teeth*, skeletal muscle, yolk sac membranes
- Ox tissues: knee (joint cartilage and bone)*
- Fish tissues: Colisa Lalia, zebrafish
- Plants: ivy leaves, carrot slices, beet roots, microcallus & callus organoids, nasturtium stamen, melon seeds, sprouted tomato seeds, rose stem slices
- Organs: mouse heart, intestine, testis, seminal gland
- Insects: firebugs (Pyrrhocoris apterus), wasps, grasshoppers, spiders, shield bugs (Graphosoma lineatum), flies, cockroaches
- Crustaceans: woodlouse
- Shell: mussel*
*for these samples, an additional step of demineralization needs to be performed before clearing.
We would greatly appreciate your valuable inputs by helping us complementing this list with any of your positive research experiences. Drop us a message through our Contact page!
Yes, it is possible to re-opacify the samples by incubating them in a 0.2 M Tris buffer at pH 8. Re-opacifying can be a practical way to select and section a region of interest for downstream assays (i.e. immunostaining).
The imaging solution has a refractive index of 1.47, matching that of the cleared sample. It is possible to increase it by modulating the dilution ratio in water as desired.
UbiClear is an aqueous solution, formulated without any organic solvent, that efficiently clears biological tissues by removing lipids and homogenizing refractive indexes of the sample's internal components.
Yes, it is possible to perform immunostaining and clear your sample with UbiClear. All immunostaining steps can either be integrated directly within the clearing protocol (in that case, sample permeabilization is not needed), or performed separately.
Immunostaining using GFP, AF405, AF488, AF555, AF647, Cy3, TMR, AM-cyan & Td Tomato have been successfully performed on samples cleared with UbiClear.
Yes, endogenous fluorescence is preserved upon clearing (this has been shown with dT-tomato and GFP). If endogenous fluorescence needs to be visualized, we recommend not performing the depigmentation step beforehand.
Yes, UbiClear can be combined with direct dyes for visualizing cellular structures. This has been validated with Evans blue and albumin-FITC for imaging blood vessels in cleared samples. UbiClear is not compatible with common direct nucleic acid dyes (DAPI, BET, methyl green, To-pro-3). If DNA staining is expected, we recommend using indirect antibody labeling strategies.
Once cleared, samples can be imaged using conventional confocal microscopes (for samples up to ~500 µm thick). Thicker samples can be imaged using light-sheet microscopes.
Need some advice? We would be happy to answer any questions on the choice of microscopy technique, data analysis and handling. Feel free to reach out at [email protected].
Total duration will vary depending on the sample thickness and internal properties. As a general rule, the total procedure should not take more than 2 hours for samples up to 1 mm thick. The protocol might be extended up to a few hours for entire organisms.
As UbiClear works by removing lipids from the samples, this solution will not be suitable for imaging & staining lipids. It is also not compatible with direct nucleic acid dyes (DAPI, BET, methyl green). If DNA staining is expected, we recommend using indirect antibody labeling strategies.
Sample shrinkage has been observed within the first few minutes of incubation in the clearing solution, which is explained by the difference in osmolarity between the sample and the solution leading to dehydration. This effect is then attenuated after a few minutes as the clearing solution penetrates inside the structures.
No, UbiClear does not contain any organic solvents neither volatile compounds. Its handling does not require the use of a chemical hood or sophisticated ventilation systems for imaging.
The UbiClear kits include a Depigmentation Buffer, a delipidation solution (Mix 1) and a solution to homogenize refractive indexes in the sample (Mix 2). These two mixes will serve to make the final clearing and imaging solutions. Everything you need to have on your end is some distilled water, 0.2M Tris at pH 8 and some 30% hydrogen peroxyde.