- All Products
- GPC4-Nanobody - stem cell differentiation booster
GPC4-Nanobody - stem cell differentiation booster
https://www.idylle-labs.com/shop/gpc4-nanobody-stem-cell-differentiation-booster-678 https://www.idylle-labs.com/web/image/product.template/678/image_1920?unique=9f93cecSingle-domain antagonizing antibody blocking Glypican-4 protein activity and promoting stem cell entry into differentiated lineages
A technology developed by Rosanna Dono (Institut du Biologie du Développement de Marseille IBDM, Aix-Marseille Université, France)
GPC4-Nanobody is a single-domain antagonizing antibody (sdAb) that recognizes and binds a conformational epitope of the native human Glypican-4 (hGPC4) with high affinity and specificity. When added to the culture medium of live cells, GPC4-Nanobody blocks hGPC4 biological functions.
GPC4-Nanobody can be used as a differentiation booster: by maintaining reduced hGPC4 levels throughout differentiation, GPC4-Nanobody addition in the differentiation medium leads to more efficient differentiated lineage entry in response to triggering factors.
GPC4-Nanobody at a glance
A differentiation booster
GPC4-Nanobody arises from the groundbreaking discovery that constant reduction in hGPC4 levels in differentiating hiPSCs makes them more responsive to differentiation signals (cf Publications section). Continuous exposure of hiPSC to GPC4-Nanobody allows them to acquire a unique biologial state characterized by:
- Maintenance of pluripotency in undifferentiated conditions (absence of differentiation factors)
- Enhanced differentiation yields upon differentiation exposure
- Reduced tumorigenicity in xenografts
Applications
GPC4 nanobody can be added directly into the culture medium to:
-> Increase differentiation yields of human stem cells for mechanistic studies or subsequent in vivo transplantation
So far, GPC4-Nanobody has been shown to increase differentiation yield of hiPSC into endoderm progenitors and dopaminergic neurons.
-> Detect hGPC4
GPC4-Nanobody can be used to detect and quantify hGPC4 levels (native or recombinant) with high specificity in immunostaining, flow cytometry or immunoprecipitation assays performed unfer non-denaturing conditions.
This nanobody binds to hGPC4 only in its native conformation: it cannot be used for hGPC4 detection in Western Blots or other assays performed under denaturing conditions.
-> Block hGPC4
GPC4-Nanobody binds hGPC4 with high affinity and blocks its biological functions: beyond hGPC4 detection, it can also be used to specifically inhibit its activity temporally or permanently in live cells. A simple way to unravel hGPC4 specific roles in various biological contexts without inducing any genetic modifications in mechanistic studies or therapeutic/diagnostic tool development!
Additional documents
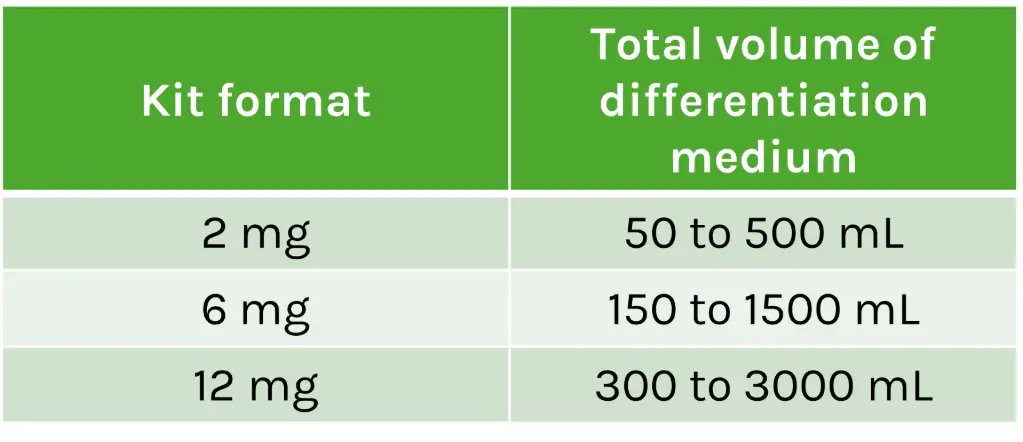
Kit contents
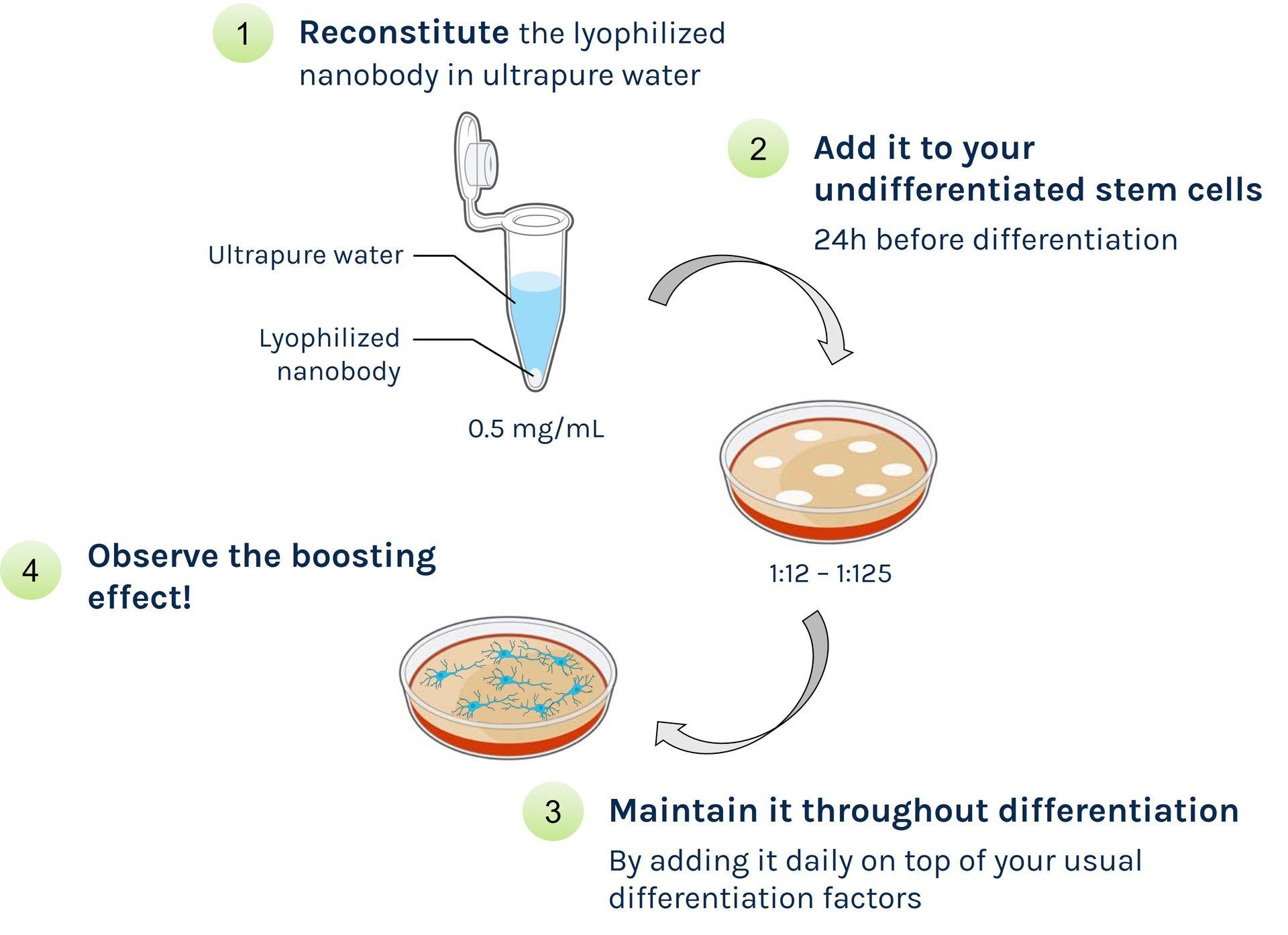
Vial contents: Lyophilized GPC4-Nanobody
Available formats: 2 mg, 6 mg or 12 mg
Storage instructions: the lyophilized nanobody can be stored at room temperature for up to one year. Once reconstituted, aliquot and freeze for long-term storage.
Recommended dilution range: 1:12 - 1:125
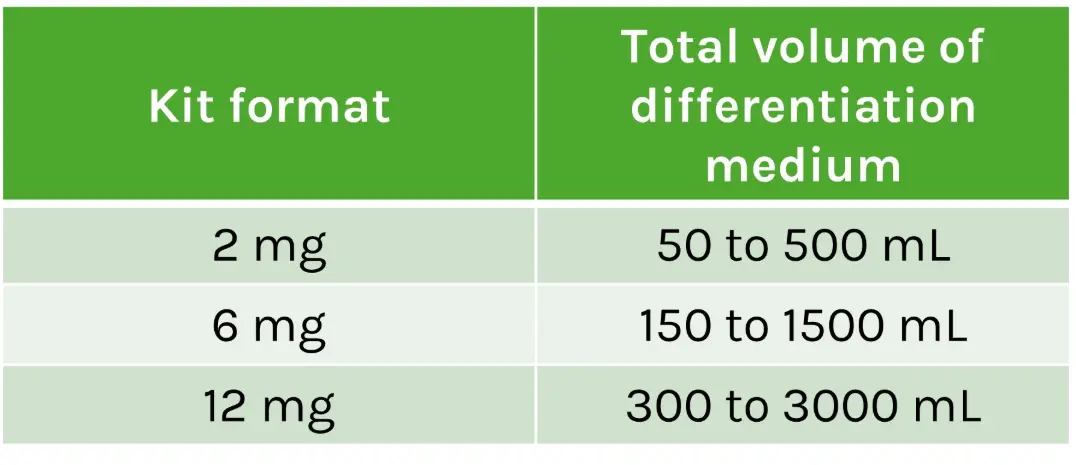
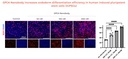
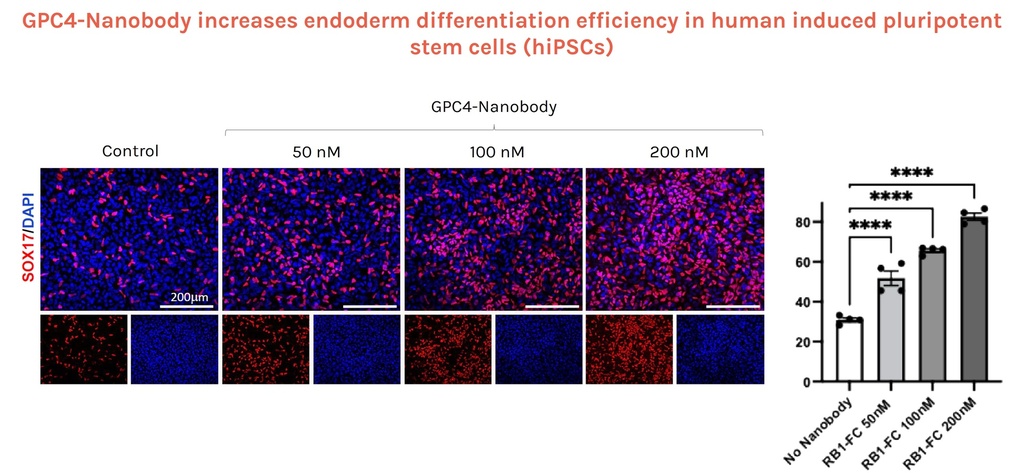
GPC4-Nanobody increases endoderm differentiation efficiency in human induced pluripotent stem cells (hiPSCs)
Left: Immunofluorescence analysis of SOX17 endodermal marker (red) and DAPI (blue) positive
cells in hiPSC cultured for 2 days with endodermal differentiation factors alone (Control) or added with GPC4-Nanobody at increasing concentrations.
Right: Quantification of the percentage of SOX17+ progenitors over DAPI+ (n = 4 biological replicates).
Credits: Rosanna Dono (Institut du Biologie du Développement de Marseille IBDM, Aix-Marseille Université, France)
Downregulation of GPC4 in hiPSCs promotes efficient generation of ventral midbrain dopamine neuron progenitors in vitro
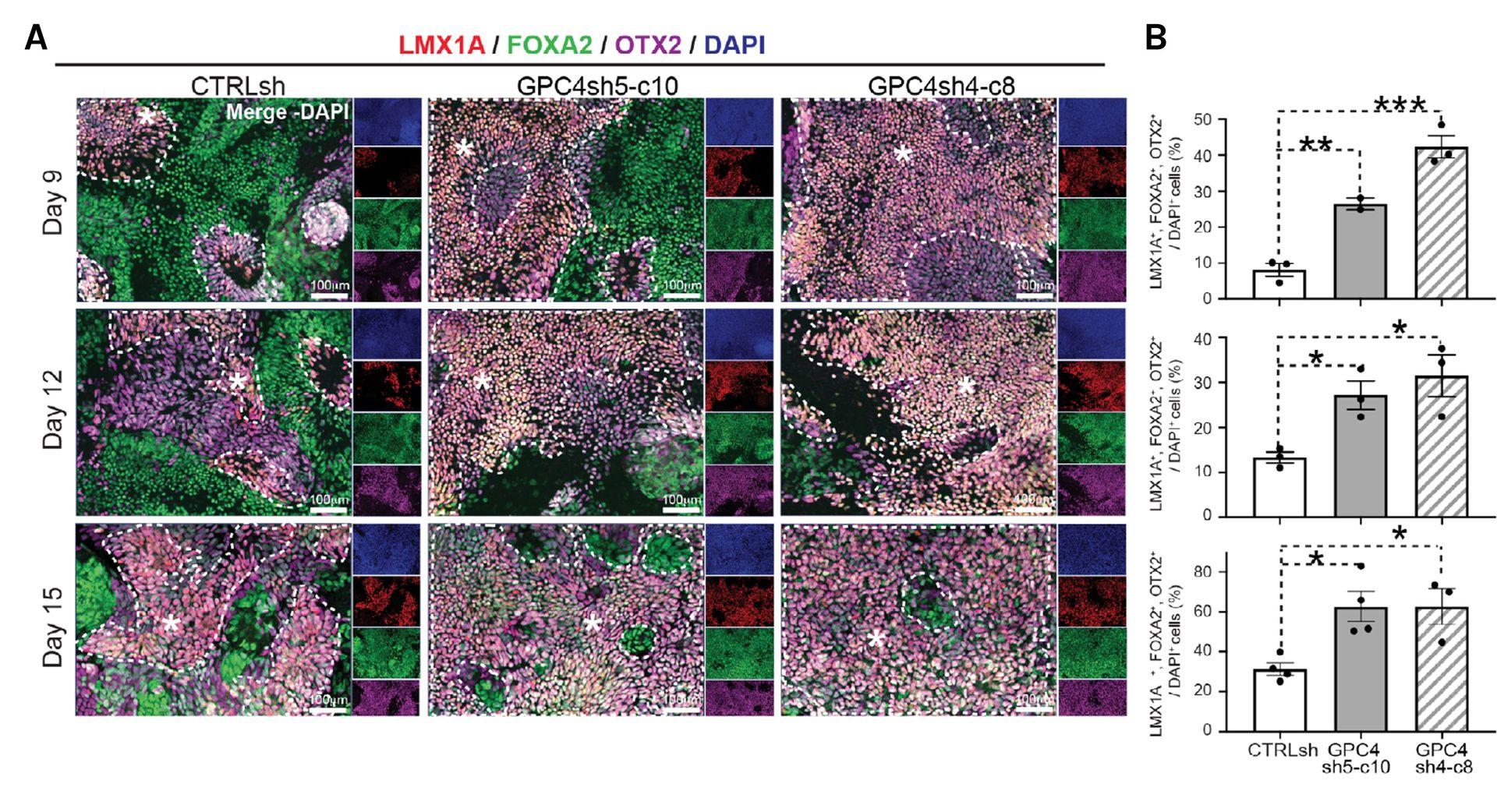
A. Immunofluorescence analysis of LMX1A (red), FOXA2 (green), OTX2 (purple), and DAPI (blue) positive
cells in CTRLsh, GPC4sh5-c10, and GPC4sh4-c8 cultures at the indicated time points of VMDA differentiation. LMX1A+, FOXA2+, OTX2+ cells,
which correspond to bona fide VMDA progenitors, are highlighted by dashed areas with a star. B. Quantification of the percentage of LMX1A+,
FOXA2+, OTX2+ VMDA progenitors over DAPI+ (VMDA progenitors at d9: CTRLsh: 8.1 ± 1.8%; GPC4sh5-c10: 26.5 ± 1.6%; GPC4sh4-c8:
42.3 ± 3.1%; at d12: CTRLsh: 13.3 ± 1.2%; GPC4sh5-c10: 27.1 ± 3.1%; GPC4sh4-c8: 31.4 ± 4.6%; at d15: CRTLsh: 31.4 ± 3.1%; GPC4sh5-c10:
62.7 ± 7.6%; GPC4sh4-c8: 62.7 ± 9.0%). Data are presented as mean ± SEM (n = 2-3 biological replicates). One-way ANOVA: *P < .05, **P < .01,
***P < .001.
Source publication: Corti S. et al, 2020
Downregulation of GPC4 in hiPSCs enhances differentiation potential into definitive endoderm
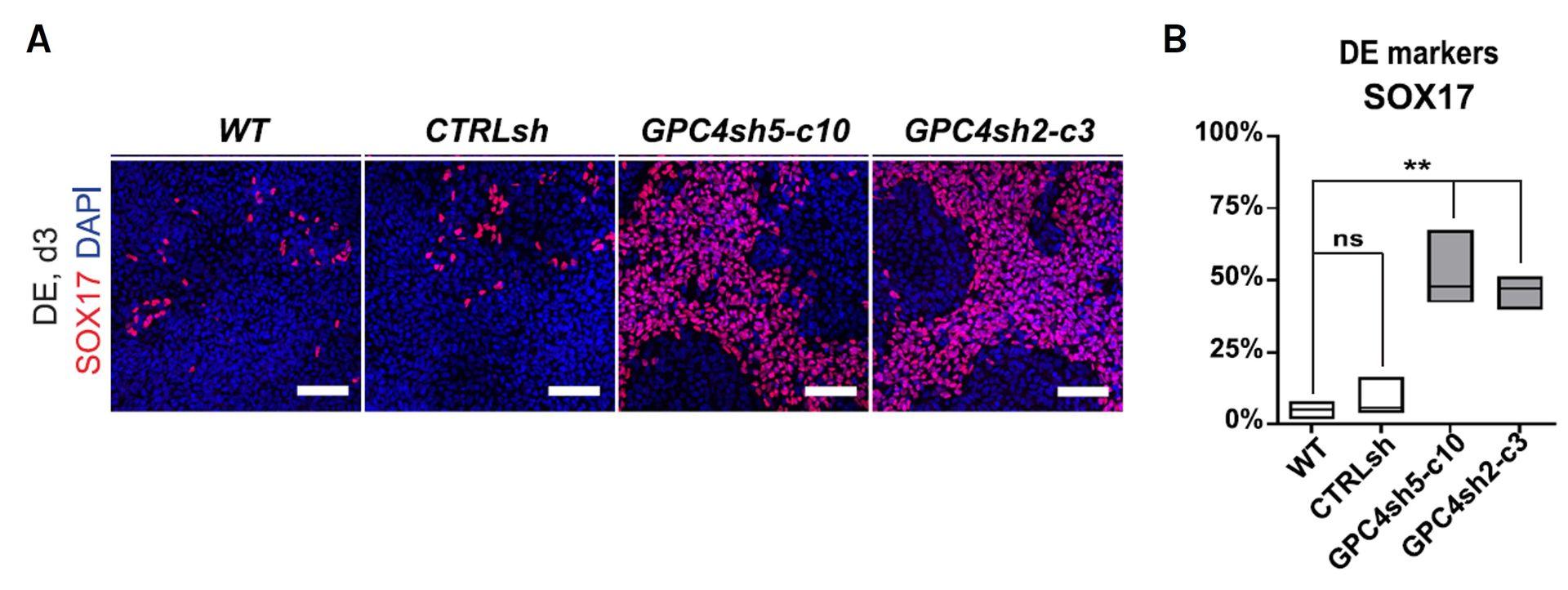
A. Immunofluorescence analysis of SOX17 (red, DE) onWT, CTRLsh, GPC4sh5-c10 and GPC4sh2-c3 029 hiPSCs differentiated for 3 days into DE, n = 3. Scale bar: 100 μm. B. Percentages of SOX17 positive cells in WT, CTRLsh, GPC4sh5-c10 and GPC4sh2-c3 029 hiPSCs were quantified from staining shown in A. Box plots represent the median with min and max values, n = 3. Statistical analysis: two-way ANOVA, followed by Dunnett’s multiple comparison test. P-values: (***) <0.001, (**) <0.01, (*) <0.05, ns not significant.
Source publication: Legier et al, 2023
So far, GPC4-Nanobody has shown significant effects on differentiation yields of hiPSC differentiated into endodermal progenitors and dopaminergic neurons.
But this boosting effect is not be specific to a particular lineage and should work with any differentiation protocol: by downregulating GPC4 activity, GPC4-Nanobody acts on the physical organization of the stem cell monolayer by making stem cells more exposed to any differentiation factors present in their surrounding medium.
The boosting effects of GPC4 downregulation have been demonstrated on a variety of differentiated cell types from the endoderm, ectoderm and mesoderm lineages (cf Results and Publications sections).
Blocking GPC4 throughout the differentiation process increases stem cells' surface of contact with the differentiation medium. Therefore, stem cells are physically more exposed to any differentiation factors used in the surrounding medium, making the differentiation much more efficient and homogeneous within the cell monolayer.
Previous studies performed on hiPSCs and cancer cell types (i.e. pancreatic cancer stem cells) demonstrated that GPC4-Nanobody does not induce any significant cytotoxic effects at concentrations up to 1000 nM.
- hGPC4 detection
GPC4-Nanobody can be used to detect and quantify hGPC4 levels (native or recombinant) with high specificity in immunostaining, flow cytometry or immunoprecipitation assays performed unfer non-denaturing conditions.
This nanobody binds to hGPC4 only in its native conformation: it cannot be used for hGPC4 detection in Western Blots or other assays performed under denaturing conditions.
- hGPC4 blocking
GPC4-Nanobody binds hGPC4 with high affinity and blocks its biological functions: beyond hGPC4 detection, it can also be used to specifically inhibit its activity temporally or permanently in live cells. A simple way to unravel hGPC4 specific roles in various biological contexts without inducing any genetic modifications!
Western blot and immunoprecipitation assays demonstrated that RB1-Fc binds to hGPC4 only in its native conformation, which strongly suggests that it recognizes a conformational epitope likely located in the core protein of hGPC4. Future structural studies will be essential to precisely define the epitope recognized by RB1-Fc.
Latest results suggest that this nanobody might act as an orthosteric competitor or an allosteric negative modulator. This is actually the first reported antibody that directly blocks hGPC4 activity: in comparison, surfen or heparin mimetics often lack specificity, potentially leading to unwanted impacts on diverse cellular functions. Soluble hGPC4 ectodomains, which can be used in some systems as a decoy to compete with endogenous GPC4 for ligand binding, do not block GPC4 per se but modulate its pathways.
Facing the high heterogeneity of hiPSC lines, some tests were performed in order to validate that the boosting effect of GPC4-Nanobody on stem cell differentiation was consistent accross independent hiPSC lines.
Although the observed increase in differentiation yield is intrinsically linked to the differentiation potential of stem cells, significant boosting effects were observed with both 029 and KOLF hiPSC lines (from 30% to 80% with the 029 line, and from 20% to 60% with the KOLF line). Take a look at the Results section for detailed figures.
Optimal GPC4-Nanobody concentration can vary according to the intrinsic differentiation potential of your stem cells, your differentiation protocol and the targeted increase in differentiation yield.
Significant improvements in the yield of differentiated cells can be observed at dilutions as low as 1:250 in the culture medium. This concentration may be increased up to 1:25. A dilution range in preliminary experiments can help selecting the optimal concentration to be used in further differentiation assays.
We recommend adding the GPC4-Nanobody throughout the whole differentiation, in order to achieve maximal differentiation efficiency. But of course it all depends which percentage of differentiated cells you're aiming for, and which step(s) of your own differentiation protocol need to be improved.
The most important thing is to make sure you are adding it one day before starting the differentiation and maintain it during the initial phase (typically 3-4 days). Then, whether it's best to keep it until the end of the protocol or whether you can stop using it at some stage should be determined experimentally. For example, if the challenging part is getting progenitors, then GPC4-Nanobody can be added during early phases only to maximize progenitor generation.
The GPC4-Nanobody is actually a bivalent nanobody in a Fc-fusion form (or in other words, a nanobody fragment fused to the Fc domain of human IgG1). So in order to detect it in subsequent assays, an anti-human IgG (Fc-specific) secondary antibody should be used.
For flow cytometry, one example reference is the Goat F(ab')2 Anti-Human IgG (Fc) (Beckman, cat. n°IM0550), which was successfully used in the following paper for GPC4-Nanobody detection in flow cytometry at a 1:250 dilution:
Remi Bonjean, Rossana Cuciniello, Brigitte Kerfelec, Patrick Chames, Rosanna Dono, Functional targeting of Glypican-4 by a conformation-specific single-domain antibody, Biomaterials, Volume 328, 2026, 123864, ISSN 0142-9612, https://doi.org/10.1016/j.biomaterials.2025.123864.
For immunofluorescence assays, any anti-human IgG (Fc-specific) secondary antibody that is conjugated to a photostable fluorophore (i.e. Alexa Fluor series) and already validated for immunofluorescence applications can be used.
Remi Bonjean, Rossana Cuciniello, Brigitte Kerfelec, Patrick Chames, Rosanna Dono, Functional targeting of Glypican-4 by a conformation-specific single-domain antibody,
Biomaterials, 2025, 123864, ISSN 0142-9612, https://doi.org/10.1016/j.biomaterials.2025.123864.
Legier, T., Rattier, D., Llewellyn, J. et al. Epithelial disruption drives mesendoderm differentiation in human pluripotent stem cells by enabling TGF-β protein sensing. Nat Commun 14, 349 (2023). https://doi.org/10.1038/s41467-023-35965-8
Corti S, Bonjean R, Legier T, Rattier D, Melon C, Salin P, Toso EA, Kyba M, Kerkerian-Le Goff L, Maina F, Dono R. Enhanced differentiation of human induced pluripotent stem cells toward the midbrain dopaminergic neuron lineage through GLYPICAN-4 downregulation. Stem Cells Transl Med. 2021 May;10(5):725-742. doi: 10.1002/sctm.20-0177. Epub 2021 Feb 2. PMID: 33528918; PMCID: PMC8046045.
Publication highlighted on the IBDM Institute's website: https://www.ibdm.univ-amu.fr/a-new-target-to-control-the-differentiation-and-tumorigenic-potentials-of-human-induced-pluripotent-stem-cells/
GPC4-Nanobody is a patented technology (International Publication Number: WO 2022/079270 A1)
Everspark technology has been intially developed by Karine Monier, Arnaud Favier and Christophe Place and and published in Scientific Reports:
Provost, A., Rousset, C., Bourdon, L. et al. Innovative particle standards and long-lived imaging for 2D and 3D dSTORM. Sci Rep 9, 17967 (2019). https://doi.org/10.1038/s41598-019-53528-0
Publications:
Nanoscale engagement and clusterization of Programmed death ligand 1 (PD-L1) in the membrane lipid rafts of Non-Small Cell Lung Cancer cells
Martina Ruglioni, Simone Civita, Tiziano Salvadori, Sofia Cristiani, Vittoria Carnicelli, Serena Barachini, Iacopo Petrini, Irene Nepita, Marco Castello, Alberto Diaspro, Paolo Bianchini, Barbara Storti, Ranieri Bizzarri, Stefano Fogli and Romano Danesi bioRxiv 2022.08.09.503318; doi: https://doi.org/10.1101/2022.08.09.503318
HIV-1 diverts actin debranching mechanisms for particle assembly and release in CD4 T lymphocytes
Rayane Dibsy, Erwan Bremaud, Johnson Mak, Cyril Favard, Delphine Muriaux
BioRxiv, December 16, 2022. doi. 10.1101/2022.12.15.520580
Fluorescent Polymer-AS1411-Aptamer Probe for dSTORM Super-Resolution Imaging of Endogenous Nucleolin
Fabre L, Rousset C, Monier K, Da Cruz-Boisson F, Bouvet P, Charreyre MT, Delair T, Fleury E, Favier A. Biomacromolecules. 2022 May 12. doi: 10.1021/acs.biomac.1c01706. PMID: 35549176
Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines.
Michalik S, Siegerist F, Palankar R, Franzke K, Schindler M, Reder A, Seifert U, Cammann C, Wesche J, Steil L, Hentschker C, Gesell-Salazar M, Reisinger E, Beer M, Endlich N, Greinacher A, Völker U. Haematologica. 2022 Apr 1;107(4):947-957. doi: 10.3324/haematol.2021.280154. PMID: 35045692
Metabolic biorthogonal labeling and dSTORM imaging of peptidoglycan synthesis in Streptococcus pneumoniae
Jennyfer Trouve, Oleksandr Glushonkov and Cecile Morlot
Star Protocols, December 13, 2021. doi: 10.1016/j.xpro.2021.101006
Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia.
Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U, Hentschker C, Michalik S, Steil L, Reder A, Schönborn L, Beer M, Franzke K, Büttner A, Fehse B, Stavrou EX, Rangaswamy C, Mailer RK, Englert H, Frye M, Thiele T, Kochanek S, Krutzke L, Siegerist F, Endlich N, Warkentin TE, Renné T.
Blood. 2021 Dec 2;138(22):2256-2268. doi: 10.1182/blood.2021013231. PMID: 34587242
Superresolution Microscopy of Drosophila Indirect Flight Muscle Sarcomeres.
Szikora S, Novák T, Gajdos T, Erdélyi M, Mihály J. Bio Protoc. 2020 Jun 20;10(12):e3654. doi: 10.21769/BioProtoc.3654. eCollection 2020 Jun 20. PMID: 33659324