Understanding of the underlying mechanisms governing cancer maturation, invasion and metastasis, as well as their interplay with potential treatment agents has driven the development of multiple in vitro tumoral models. Spheroids are three-dimensional (3D) cellular aggregates that can mimic tissue and microtumors. So far, spheroids could be obtained through different approaches, like the hanging drop method or matrix approaches that promote cell growth in natural three-dimensional structures, mostly microwells (usually made of collagen, fibronectin, agarose, laminin or gelatin). Here, the original method is based on the magnetic attraction of magnetized cells towards a fixed magnet, a force which ultimately drives cell aggregation into a defined shape. These magnetic spheroids accurately recapitulate the behavior of 3D cancer cell aggregates, in addition to being highly reproducible, finely tuned and having a very low maturation time. This method allowed to monitor many parameters like invasiveness, surface tension, cohesiveness and drug resistance of tumor spheroids.
Publication: Magnetic molding of tumor spheroids: emerging model for cancer screening
#MagneticTissueEngineering #TumorSpheroids #MagneticNanoparticles #CancerScreening #SurfaceTension

A blog post by Marie Tautou
I have a PhD in Neuroscience with a specialization in Pharmacology. I am passionate about discovering new treatments, and during my PhD I have studied small molecules to cure Alzheimer's disease using rodent models. I am currently the scientific and business development director of Antineo, a CRO providing preclinical expertise and services to private companies and academic institutions in the field of Oncology.
.png)
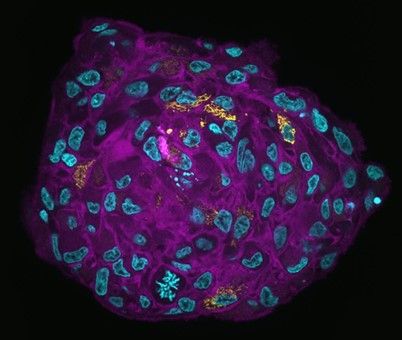
A brief introduction to spheroids
Spheroids are aggregates of cells growing in a three-dimensional manner in suspension and developing complex interactions between cells or a three-dimensional matrix (like matrigel). Their primary interest is to reproduce an architecture similar to the original tissue or organ, which will generate different nutrients gradients, gas (oxygen), growth factors, etc. Spheroids are thought to mimic tumor behavior more effectively than normal 2D cultures because, like tumors, they contain both deeply embedded and surface-exposed cells, proliferating and non-proliferating cells, and a hypoxic center with a well-oxygenated outer layer of cells. They allow visualization of the physiological changes that differentiate tumor cells from healthy cells. Spheroids are also ideal for preclinical drug screening and identify potential cancer drugs. Specifically, they can reveal the potential of drugs to infiltrate the tumor, as well as their inhibitory effects on metastasis.
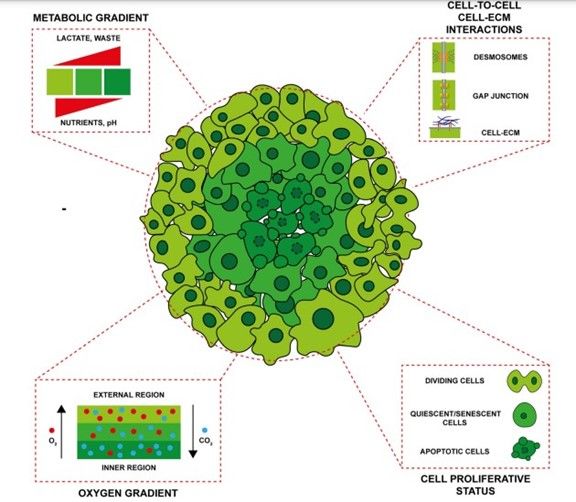 Figure 1: Main characteristics of spheroid model. Zanoni et al. Journal of Hematology & Oncology (2020)
Figure 1: Main characteristics of spheroid model. Zanoni et al. Journal of Hematology & Oncology (2020)
Compared to other 3D cell culture methods, spheroids have several advantages, including relevance to tumor biology, lower cost, reproducibility, ease of integration into high-throughput screening, and advanced imaging techniques. There are a number of distinct methods for promoting spheroid formation in culture, including hanging drop, liquid overlay, pellet culture, spinner culture, microfluidics, and methods incorporating biomaterial scaffolds like hydrogels or biofilms made of alginate, collagen, and hyaluronic acid. While the liquid overlay technique is advantageous for certain cell types, the formation of tumor spheroids is inhibited by agarose. For the spinner culture method, the sizes of the resultant spheroids are partially dependent on the size of the container, whereas for biomaterial scaffolds the set up can take a consistent time. Although the size of the spheroids can be relatively easy to control, the cell density within the spheroids is not. In addition, not all tumor cell lines can be used to generate spheroids.
The formation of magnetic spheroids with high sphericity allows the fine-tuning of the final spheroid diameter
Here the spheroid formation relies on the magnetic attraction of magnetized cells towards a fixed external magnet, with the magnetic force acting remotely to actively aggregate the cells into a spherically shaped agarose mold. This magnetic labelling method to obtain tumor-like spheroids from cancer cells with a tightly controlled sphericity and tunable diameters is biocompatible, having no detrimental effects on spheroid morphology and growth, cell proliferation, viability and invasiveness. This is a highly reproducible method, and mature spheroids can be obtained in a very short time, less than a day of incubation. In addition, the magnetic nature of the spheroids enables the monitoring of the surface tension parameter, which is of high relevance in cancer tumor cell cohesiveness and invasiveness. Moreover, the method can accommodate other cell lines able to be magnetically labelled.
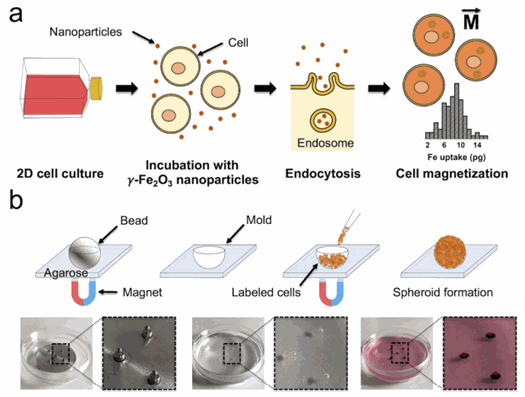 Figure 2: Cell labeling process and magnetization of cells (Perez et al, Biofabrication, 2020)
Figure 2: Cell labeling process and magnetization of cells (Perez et al, Biofabrication, 2020)This scaffold-free magnetic cell levitation method uses external magnets and magnetically labelled cells by combining it with an agarose scaffold. They used two different cancer cell lines: CT26 murine colon carcinoma and U-87 MG human glioblastoma cells. The cells are first magnetized with γ-Fe2O3 nanoparticles, of an average diameter of 8 nM. The cells are then co-incubated with magnetic nanoparticles in 2D culture, conferring them with a magnetic moment M⃗ after endocytosis. Then a spherical mold is prepared by embedding of a steel bead with a defined diameter in a layer of agarose and fixed in place with an array of 6 × 2 mm cylindrical neodymium magnets. Three different sizes of molds were used: 0.4, 0.5 and 1 mm. Finally, within minutes using the same external magnet setup, the magnetically labelled cells are seeded and aggregated into a spheroid as they are attracted by the magnetic field gradient created by each magnet. Afterwards, cohesive spheroids can be obtained in less than 24 h.
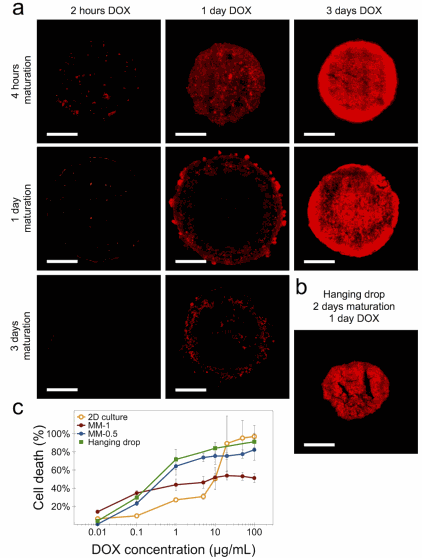 Figure 3
:
Spheroid drug resistance and penetration visualization (Perez et al, Biofabrication, 2020)
Figure 3
:
Spheroid drug resistance and penetration visualization (Perez et al, Biofabrication, 2020)
Thanks to this technique, the authors investigated the cell penetration and distribution of liposomal-encapsulated doxorubicin (DOX) in magnetically molded spheroids of 1 mm initial size. They showed that the spheroid has a different cohesiveness than a hanging drop spheroid: whereas the magnetic one is well into its maturation state, the hanging drop spheroid has barely achieved a cohesive state by this maturation time, which translates to a more uniform drug penetration profile. The cells in the 3D spheroid configuration thus show a higher resistance to DOX compared to those in 2D culture. This indicates a limited penetration of the drug towards the spheroid core.
3D magnetically shaped spheroids and their multiple potential applications
The authors showed how the spheroid maturation impacts cell invasion and doxorubicin penetrability, highlighting the importance of this parameter in drug screening and therapeutic applications, and demonstrated that this method enables to measure the surface tension of spheroids, which is particularly relevant as an output parameter in the context of cancer cell invasion and metastasis.
In conclusion, as it can be magnetically patterned into specific shapes, the design of this tool has many advantages of size-tuning and low spheroid maturation times. Magnetic labelling can also be combined with agarose well patterning and bioprinting. It can be adapted to a lot of different types of cancer cells and environments, making it a good alternative or test-experiment before going into in vivo models. The reliability and robustness of preclinical research data could certainly be enhanced by the synergistic use of this kind of 3D models, thus reducing the use of animal testing. Advances in 3D cell culture techniques thus offer the potential to both better identify drug candidates and better understand and predict the occurrence of tumor resistance to anticancer drugs.
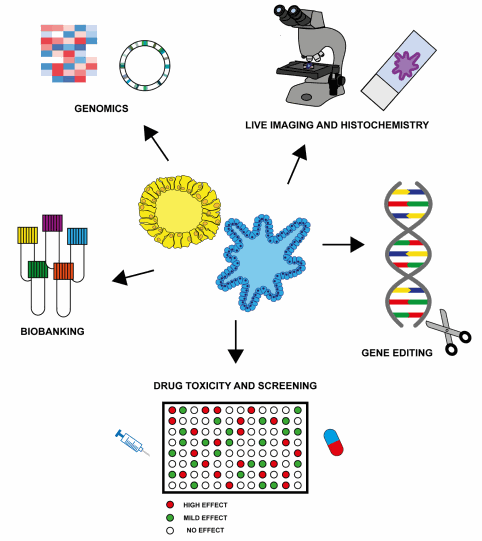 Figure 4:
Potential research and clinical applications of organoids.
Zanoni et al
.
Journal of Hematology & Oncology (2020)
Figure 4:
Potential research and clinical applications of organoids.
Zanoni et al
.
Journal of Hematology & Oncology (2020)
References:
Edwards SJ, Carannante V, Kuhnigk K, et al. High-Resolution Imaging of Tumor Spheroids and Organoids Enabled by Expansion Microscopy. Front Mol Biosci. 2020;7:208. Published 2020 Sep 24. doi:10.3389/fmolb.2020.00208
Zanoni M, Cortesi M, Zamagni A, Arienti C, Pignatta S, Tesei A. Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol. 2020;13(1):97. Published 2020 Jul 16. doi:10.1186/s13045-020-00931-0
MORE LIFE SCIENCE TOOLS TO FOLLOW
You want the latest on life science tools to follow, test and more?