Ralitza Staneva
Federica Burla
Gijsje H Koenderink
Stéphanie Descroix
Danijela Matic Vignjevic
Youmna Attieh
Marine Verhulsel
Cancer cells microenvironment is characterized by an abnormal synthesis of extracellular matrix components and an increase of matrix stiffness. That tool enabled the authors to answer the question of the importance of tissue rigidity in the chronology of tumor invasion. Indeed, reconstituted collagen I matrices can be effectively stiffened with sugar threose with no direct effect on residing cells.
Publication: A new biomimetic assay reveals the temporal role of matrix stiffening in cancer cell invasion
#CellTransformation #CollagenTypeI #ExtracellularMatrix #TumorMicroenvironment #TumorInvasiveness

A blog post by Marie Tautou
I have a PhD in Neuroscience with a specialization in Pharmacology. I am passionate about discovering new treatments, and during my PhD I have studied small molecules to cure Alzheimer's disease using rodent models. I am currently the scientific and business development director of Antineo, a CRO providing preclinical expertise and services to private companies and academic institutions in the field of Oncology.
.png)
An in vitro tool to modulate the rigidity of the extracellular matrix
Tumor progression is known to increase the extracellular matrix (ECM) stiffness, which in turn can influence tumor growth and metastasis.
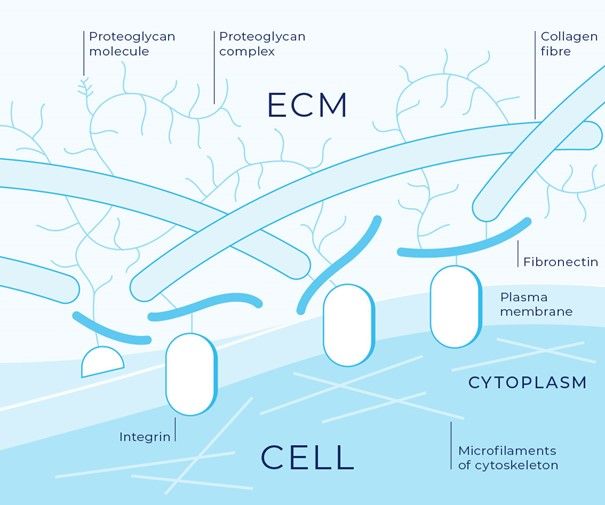 Image credit: Bonnans C. et al. Nature Reviews, 2014
Image credit: Bonnans C. et al. Nature Reviews, 2014 Therefore, it is of great interest to study the effects of tissue rigidity during tumor invasion using artificial matrixes. Traditionally, scientists used fibronectin-coated polyacrylamide gels (Butcher et al, 2009; Levental et al, 2009), or ribose to stiffens collagen matrixes, which only works at high concentration and can disturb the cells. These solutions to control tissue rigidity are scarce, do not allow to change the rigidity dynamically and are complex to implement.
To overcome this issue, the authors developed this new tool that allows to modify matrix stiffness alone at different time points during tumor invasion. Threose-stiffened Collagen I was chosen for their artificial matrix, as it supports the growth of cells in three dimensions while closely mimicking in vivo fibrillar extracellular membranes and reproducing cell–matrix interactions specific to tumors.
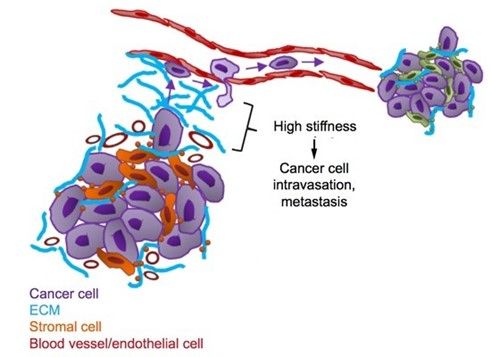
Figure 1: The tumor matrix typically consists of excessive levels of fibrous collagen
Low concentration of threose is effective in changing the stiffness of collagen gels
The authors used threose, a sugar and natural collagen cross-linker that is reported to be more effective than the traditionally used agent ribose. They incubated collagen gels with threose after polymerization to avoid any interference with fibrillogenesis and minimize potential changes in network architecture. They also analysed when to inject the threose so the fibrillogenesis of the collagen would not be disturbed.
Figure 2: Threose is effective at lower concentrations than ribose, which is advantageous, as lower sugar concentrations diminish the likelihood to induce a diabetic phenotype or cause a hypertonic stress to cells (Staneva et al).
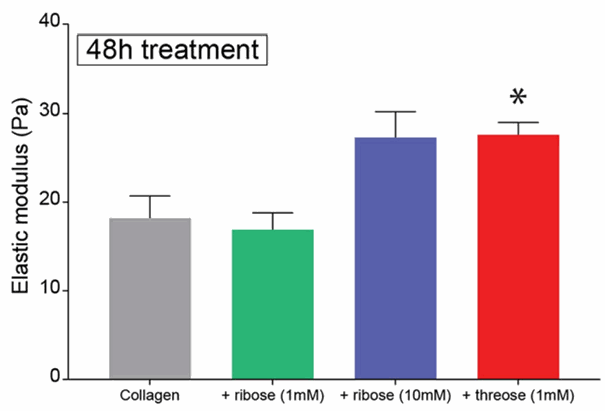 Image credit: Staneva et al, 2018
Image credit: Staneva et al, 2018
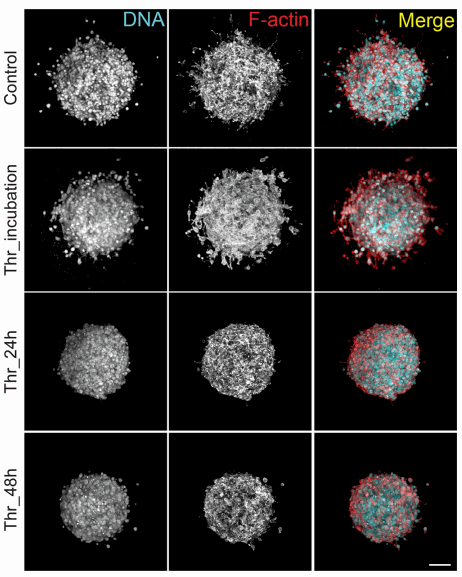 Image credit: Staneva et al, 2018
Image credit: Staneva et al, 2018
A tool that allows to study the effect of matrix stiffness
This technique is particularly interesting because it mimics in vitro a physiological tumor model composed of spheroids embedded in three-dimensional collagen matrices, that the authors were able to stiffen thanks to a natural cross-linker, threose. This in vitro model allowed them to directly test the correlation between matrix stiffness and tumor aggressiveness observed in patients.
Figure 3
:
Thanks to this technique, it was showed that matrix remodeling is necessary for cancer cells to migrate out of the spheroid and that collagen stiffening before the onset of invasion inhibits cancer cell invasion (Staneva et al).
The design of this tool is great because
it allowed to show the importance of the chronology of events in cancer cell invasion, and that collagen organization at the onset of invasion plays a critical role in the outcome of tumor development. More importantly, this device was able to capture the complexity of cancer invasion by introducing a fourth dimension into cell biological models! This could also be a great tool to study the influence of tumor stiffness on the formation of metastasis, how the ECM respond to high stiffness, the processes of intravasation and extravasation, or to test therapeutics.
It can be adapted to a lot of different types of cancer cells, levels of aggressiveness and environments, making it a good alternative or test-experiment before going into in vivo models.
Figure 4: However, at later stages of tumor development, matrix stiffening enhances cell migration, resulting in extensive invasion (Adapted of Reid et al, 2017)
References:
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801. doi:10.1038/nrm3904
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. doi:10.1016/j.cell.2009.10.027
Reid SE, Kay EJ, Neilson LJ, et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017;36(16):2373-2389. doi:10.15252/embj.201694912
MORE LIFE SCIENCE TOOLS TO FOLLOW
You want the latest on life science tools to follow, test and more?