Viruses have become essential neuroanatomical tract-tracing tools to understand brain anatomy and functions. Scientists have used different virus strains and mutations to adapt the viral properties to their needs. One member of the rhabdovirus family, the rabies virus, appears to be the most efficient and specific to identify an entire neuronal network, up to its sources.
The publication where I discovered it:
Viruses in connectomics: Viral transneuronal tracers and genetically modified recombinants as neuroscience research tools. In Journal of Neuroscience Methods (Vol. 346)
#HerpesSimplex #Pseudorabies #RabiesVirus #VesicularStomatitisVirus #Transneuronal #Connectomics

A blog post by Hélène Vitet
I have a PhD in neuroscience with an engineering background, this might be why I am interested in combining biology and technology. During my PhD, I have studied intracellular dynamics in neurons using microfluidics and I am currently pursuing a postdoc focusing on circadian rhythm establishment in the brain. Writing on the Idylle blog is more than just a way to keep me updated about new innovative tools, it’s about sharing and promoting state-of-the-art techniques that deserve to get your attention.
.png)
Back in the 1980's, when scientists started to develop tools to understand the brain organization.
More precisely, they wanted to understand the way brain structures are connected to each other through tracts: neuroanatomical tract-tracing tools. A tracer that would be considered as efficient would gather these six properties (figure 1):
1) The transfer of the tracer must be specific; from one neuron to another one through the synapse (transneuronal transfer)
2) The transport of the tracer must be unidirectional
3) The tracer must label all groups of neurons involved in the signal pathway
4) The tracer must identify the different synaptic steps
5) The tracer should not affect the cell physiology
6) The tracer should be stable in time.
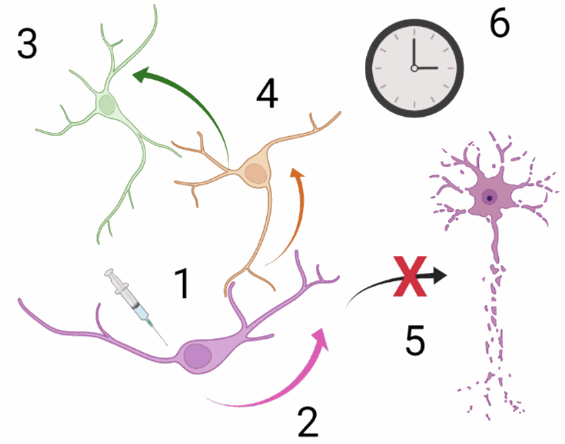
Figure 1: six properties for a good neuronal tracer to study the signal inputs and neuronal network. Credits: Hélène Vitet with Biorender
The first one that they developed, the tritium-labeled proline, lacked specificity because of the passive transfer between cells.
Then, the WGA-HRP (Wheat Germ Agglutinin- horseradish peroxidase) or the TTF (tetanus toxin fragments) could label order two neurons but were inefficient, bidirectional, and detrimental to the cells.
Then, new strategies based on viral applications were discovered and implemented. Two of them are based on the α-herpes virus (HSV-1) and Rhabdovirus (RABV). They both use cellular endocytosis and transport machineries for transneuronal transfer. The HSV-1 can label at least two synapses with neurons or other cell types but is transported bidirectionally and induces cytopathic changes, triggers an inflammatory response and rapid degeneration. On the contrary, the RABV seems to recapitulate the six main properties of a good tracer (figure 2).
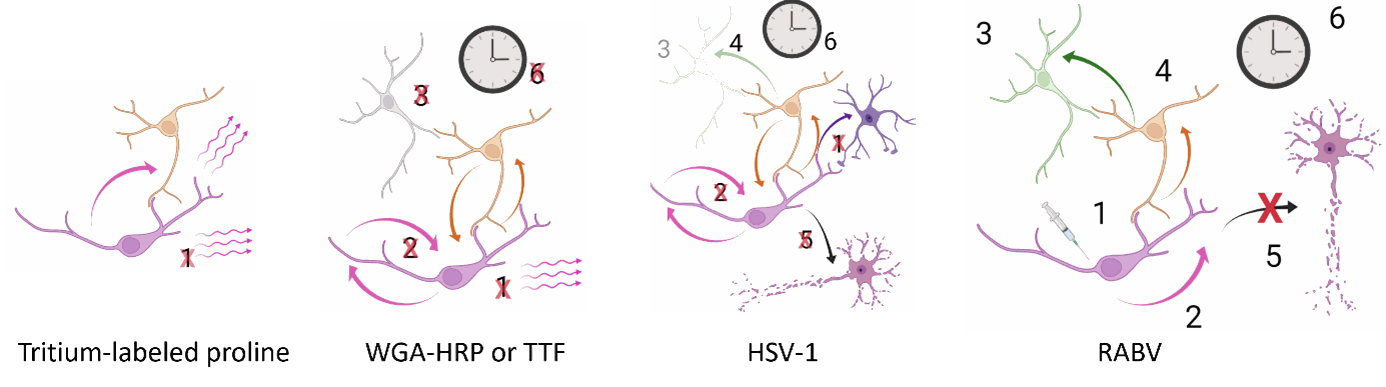
Figure 2: properties of different tracers. Credits: Hélène Vitet with Biorender
Why should you use rabies?
Billions of neurons are interconnected throughout the brain, and it can be difficult to decipher which neurons are involved in a specific network. This tool deserves our attention because it is the perfect tool for connectomics studies. Indeed, its specific retrograde transneuronal transfer allows polysynaptic tracing without any spurious labeling. The rabies virus labels an unlimited number of neurons along with the neuronal network without affecting the cell physiology. Moreover, its labeling is stable, and its kinetics properties identify the different synaptic steps (figure 1). Finally, it has a wide host range in mammals and can be combined with other molecular tools like CTB to visualize the injection site.
How do rabies outperform other neural tracers?
Rabies virus solves many problems you could have encountered thanks to its properties.
1) Labelling the neuronal cells involved in a specific network: its ubiquitous receptors on neuronal cells across CNS allows the specific transneuronal transfer (figure 3).
2) Discriminating the projections from the signal source: rabies virus can bind only to the retrograde molecular motor (dynein), allowing the unidirectional and retrograde transport of the virus (figure 3).
3) Labelling the entire network: the virus is fully competent for replication, so it can label an unlimited number of synapses (figure 4)
4) Identifying the synaptic steps: its slow kinetics allows to label another neuron order every 12 hours.
5) Preserving the cell integrity: its “hide and seek” strategy, developed to escape the immune response, preserves the cell integrity.
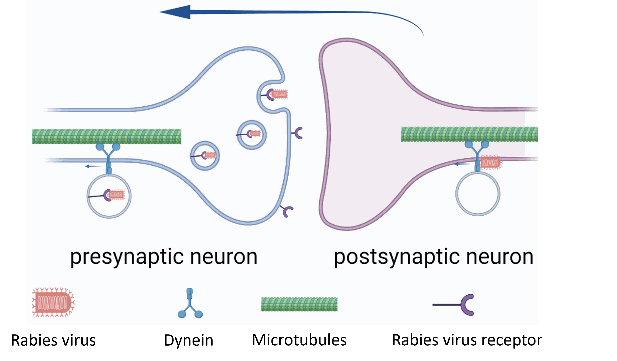
Figure 3: rabies virus transneuronal transfer through receptor binding and retrograde transport. Credits: Hélène Vitet with Biorender
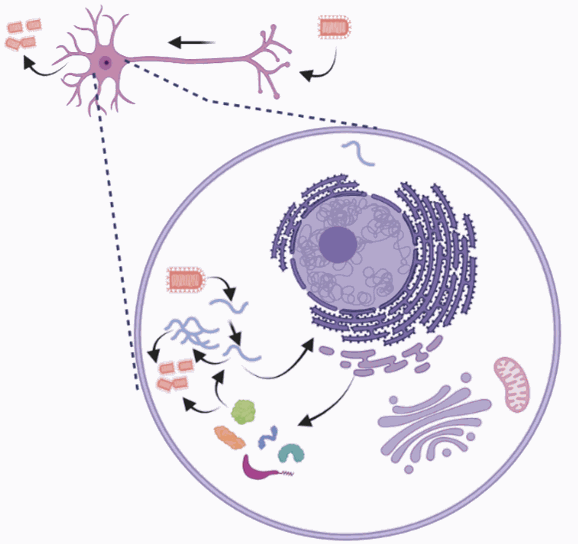
Figure 4: rabies virus self-replicates in the neuronal cells. Credits: Hélène Vitet with Biorender
What can you use it for?
Rabies virus will help you to investigate your biological question from another point of view; instead of studying the impact of a signal on neuronal projections, you can understand its sources (figure 5). This way, you can modulate it in a more integrated way.
In terms of application, RABV is ideal to study motor innervation since it is injected through intramuscular injections.
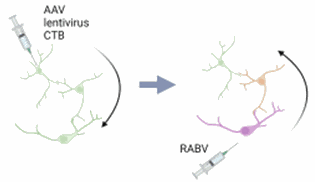
Figure 5: from studying neuronal projection to studying neuronal inputs: the rabies virus changes your point of view. Credits: Hélène Vitet with Biorender
In a nutshell
Many studies focus on the anterograde transport of the nervous signal and often study the downstream impact of their neuronal manipulation. But in such an integrated organ that is the brain, it is important to understand what comes upstream. For this purpose, the rabies virus, as a retrograde tracing tool, appears to be the ideal method.
MORE LIFE SCIENCE TOOLS TO FOLLOW
You want the latest on life science tools to follow, test and more?