Fazil Emre Uslu
Christopher D. Davidson
Erik Mailand
Ghislaine Caillol
Nikolaos Bouklas
Brendon Baker
Mahmut Selman Sakar
Cell culture environment both physiologically relevant and that allows measurement and exertion of forces are challenging to develop. In this work from Uslu and colleagues, a clever design embeds both a fibrillar extracellular matrix and a way to reliably generate and measure stresses.
Publication:
Engineered Extracellular Matrices with Integrated Wireless Microactuators to Study Mechanobiology, Advanced Materials, Volume 33, Issue 40, 2102641
#ECM #MagneticActuator #Mechanobiology #TissueEngineering

A blog post by Adrien Méry
After my engineering degree, I spent my PhD exploring the mechanical response of tissues using optogenetics and tissues engineering. This was also when I developed a passion for science communication and started working as a freelance scientific copywriter. Taking part to the Idylle blog is thus the perfect opportunity to combine both my love for pop-sci and cool research tools.
.png)
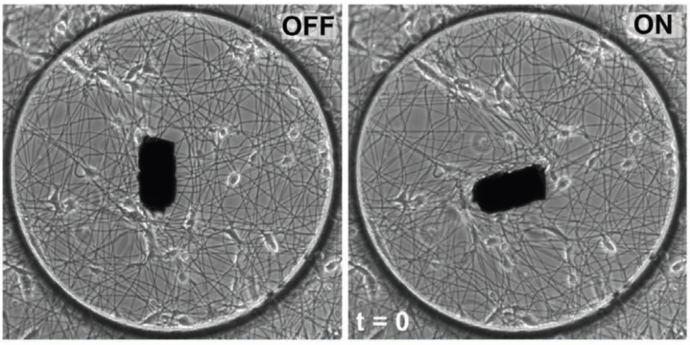
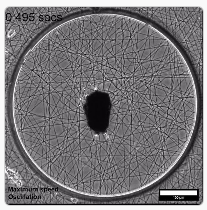
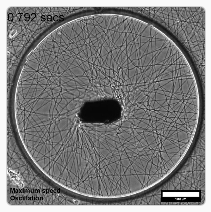
Figure 1 The MICROBS team from EPFL developped a magnetic actuated ECM environment to exposed cells to physiological stretching. Adapted from Uslu, Advanced Materials, 2022, 10.1002/adma.202102641
The mechanobiology quest
It has been known for decades most cells can perceive mechanically. Mechanobiology put to task to identify what proteins and signaling pathways were involved in this perception, and what response cells would generate. The seminal experiments resorted to in vitro setups to stretch, compress, shear cells. But as the field progressed, and the tools available boomed, mechanobiologists yearned for more and more physiologically relevant setups: they used 3D fibrillar environments, with chemical compositions closer to what is found in living systems. With this complexification of the experimental setups, keeping ways to access the stress applied to the cells has become more and more challenging.
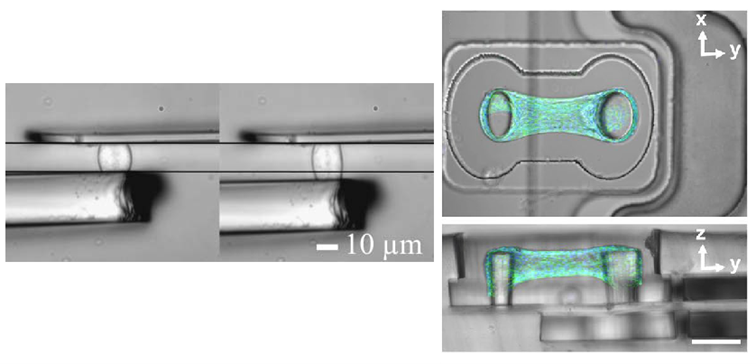
Figure 2: On the left, a simple cell stretcher using microplates in 2005 (adapted from Desprat, Biophysical Journal, 2005, 10.1529/biophysj.104.050278). On the right, a microtissue assembled on a vacuum actuated stretcher. The tissue is made of hundreds of cells and collagen. The actin is marked in green and the nuclei in blue. The scale bar is 100 µm (adapted from Walker, Scientific Report, 2020, 10.1038/s41598-020-64725-7).
A web and a magnet
To address these requirements, MICROBS team in EPFL proposed a clever design. As a substrate for the cells, they electrospun dextran fibers to form a web with mechanical properties that imitates the extracellular matrix. A paramagnetic element is glued at the center of the web and can be controlled by a matrix of magnets around the setup: rotating this matrix applies a torque on the actuator on the web, twisting it. Using a numerical clone of the web (obtained from a simple picture), the forces applied on the fibers are locally estimated.
The magnetic actuation has a quick response time and produces forces comparable to cellular forces. As a proof of concept, the publication reports a faster and more persistent migration of fibroblasts on an actuated web exerting forces close to the one the cells would. This system can thus be used to explore the dynamical response of cells due to fibers recruitment over distances.
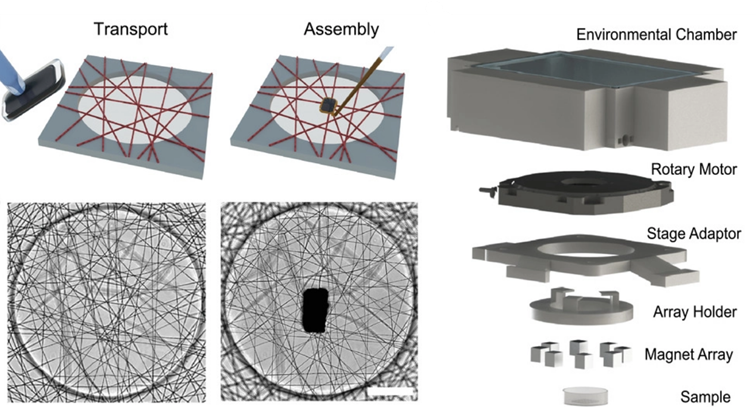
Figure 3: Assembly of the actuator and the web on the left (scale bar is 100 µm) and 3D view of the 3D actuation system. Adapted from Uslu, Advanced Materials, 2022, 10.1002/adma.202102641
A simple yet promising tool
In the field of mechanobiology, experiments tend to become more and more elaborate and intricate, to perform the mechanical measurement in an environment as natural for the cells as possible. In contrast, this setup kept things as simple as possible, using only one actuator on a 2D web well characterized. And yet, the team did a significant step in the direction of the physiological relevance. Despite bringing a fibrillar environment for the cells, the easier to calibration than a bio extracted matrix which makes experiments more reproducible. Overall, the team of Sakar brought an elegant tool to the community, that could become a reference to better understand long range cell interactions through fibrillar environments.
References:
L. Li, J. Eyckmans, and C. S. Chen, “Designer biomaterials for mechanobiology,” Nature Materials, vol. 16, no. 12. Springer Science and Business Media LLC, pp. 1164–1168, Dec. 01, 2017. doi: 10.1038/nmat5049
S. Kim, M. Uroz, J. L. Bays, and C. S. Chen, “Harnessing Mechanobiology for Tissue Engineering,” Developmental Cell, vol. 56, no. 2. Elsevier BV, pp. 180–191, Jan. 2021. doi: 10.1016/j.devcel.2020.12.017
M. Walker, P. Rizzuto, M. Godin, and A. E. Pelling, “Structural and mechanical remodeling of the cytoskeleton maintains tensional homeostasis in 3D microtissues under acute dynamic stretch,” Scientific Reports, vol. 10, no. 1. Springer Science and Business Media LLC, May 06, 2020. doi: 10.1038/s41598-020-64725-7
MORE LIFE SCIENCE TOOLS TO FOLLOW
You want the latest on life science tools to follow, test and more?