- All Products
- a-Clipse - Chambers for luminescence imaging and photo/spectro-electrochemistry
a-Clipse - Chambers for luminescence imaging and photo/spectro-electrochemistry
https://www.idylle-labs.com/shop/a-clipse-chambers-for-luminescence-imaging-and-photo-spectro-electrochemistry-500 https://www.idylle-labs.com/web/image/product.template/500/image_1920?unique=a35f42dAdaptative Chambers for Luminescence Imaging and Photo/Spectro-Electrochemistry
A technology developed by Bertrand Goudeau & Stephane Reculusa
(Institute of Molecular Sciences - Bordeaux, France)
Monitoring electrochemical reactions in situ can prove difficult.
a-Clipse is a set of adaptative chambers, implementable on commonly used microscope and spectrometer instruments, that offer the possibility to conciliate electrochemical reactions and microscopic/spectroscopic readouts in a safe, stable and controlled environment.
a-Clipse at a glance
The a-Clipse chambers have been specifically designed to make the most of your electrochemical experiments:
in situ monitoring
Allows dynamic in situ tracking of electrochemical processes as they occur
Minimal reagents
Requiring minimal sample/reagent amounts, they are ideal for precious or rare materials
Precise control
Experimental conditions are precisely controlled and maintained in an air-tight container, minimizing sample disturbance and improving reproducibility
Compatible with dynamic assays
Adaptable flow system allowing to work in dynamic conditions
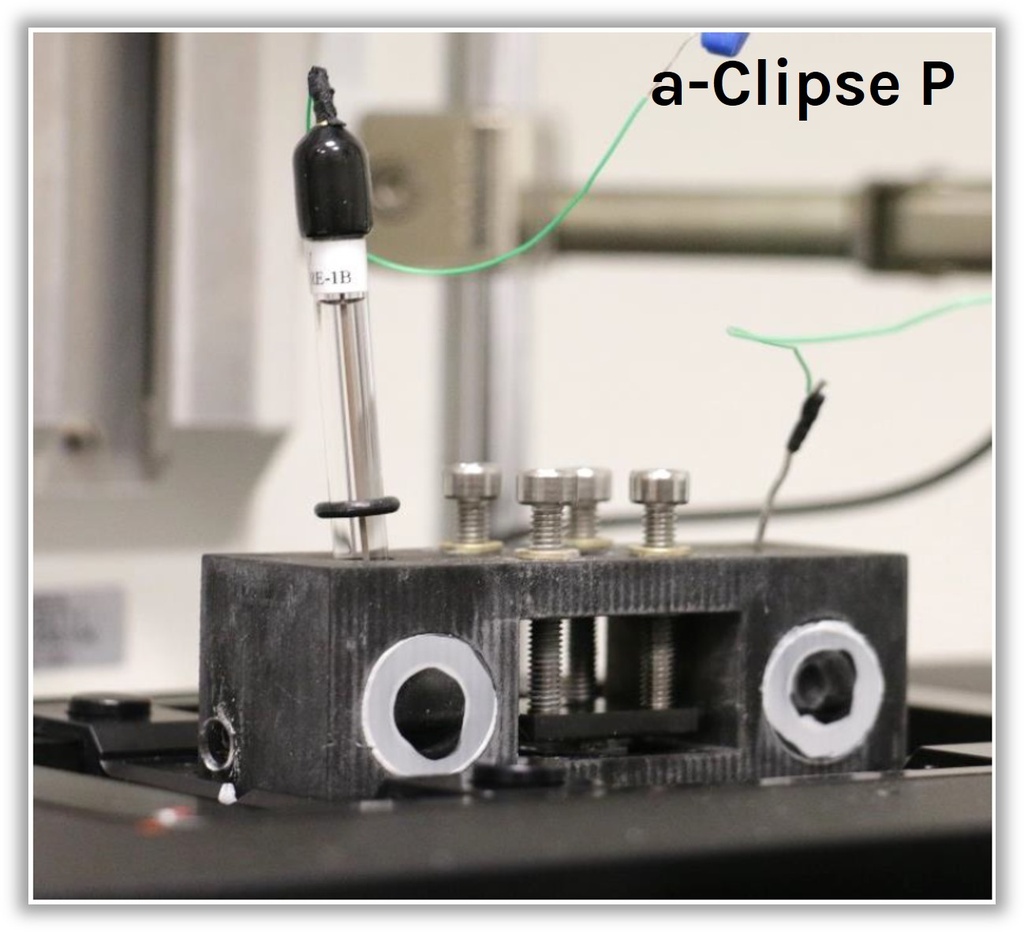
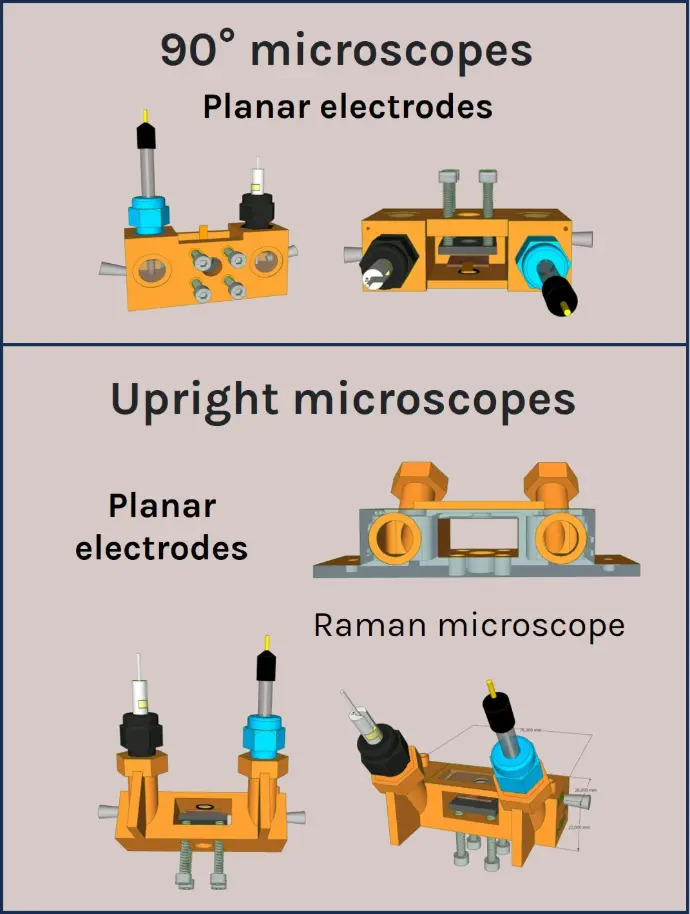
a-Clipse P conceived for inverted microscopes
Ready to take your electrochemical experiments to the next level?
Product specifications
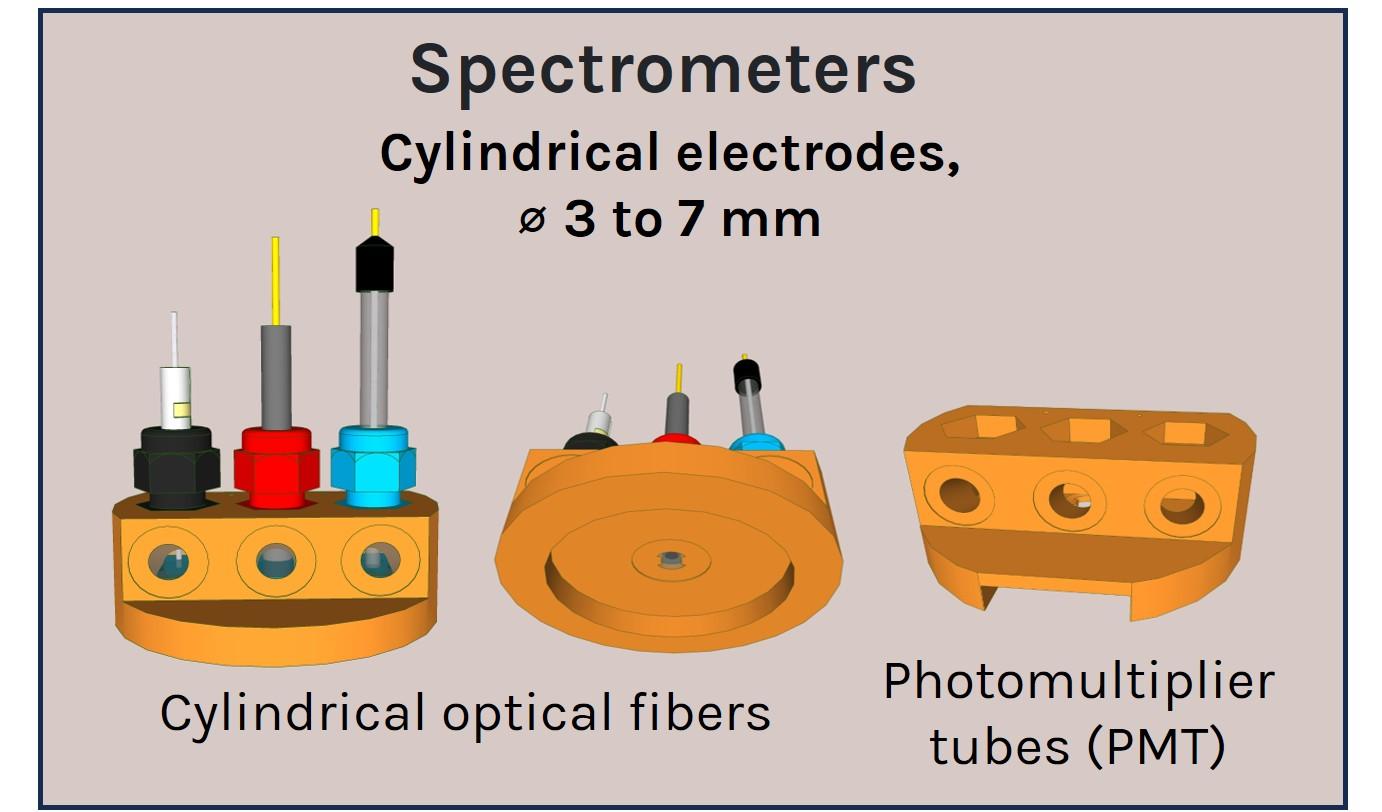
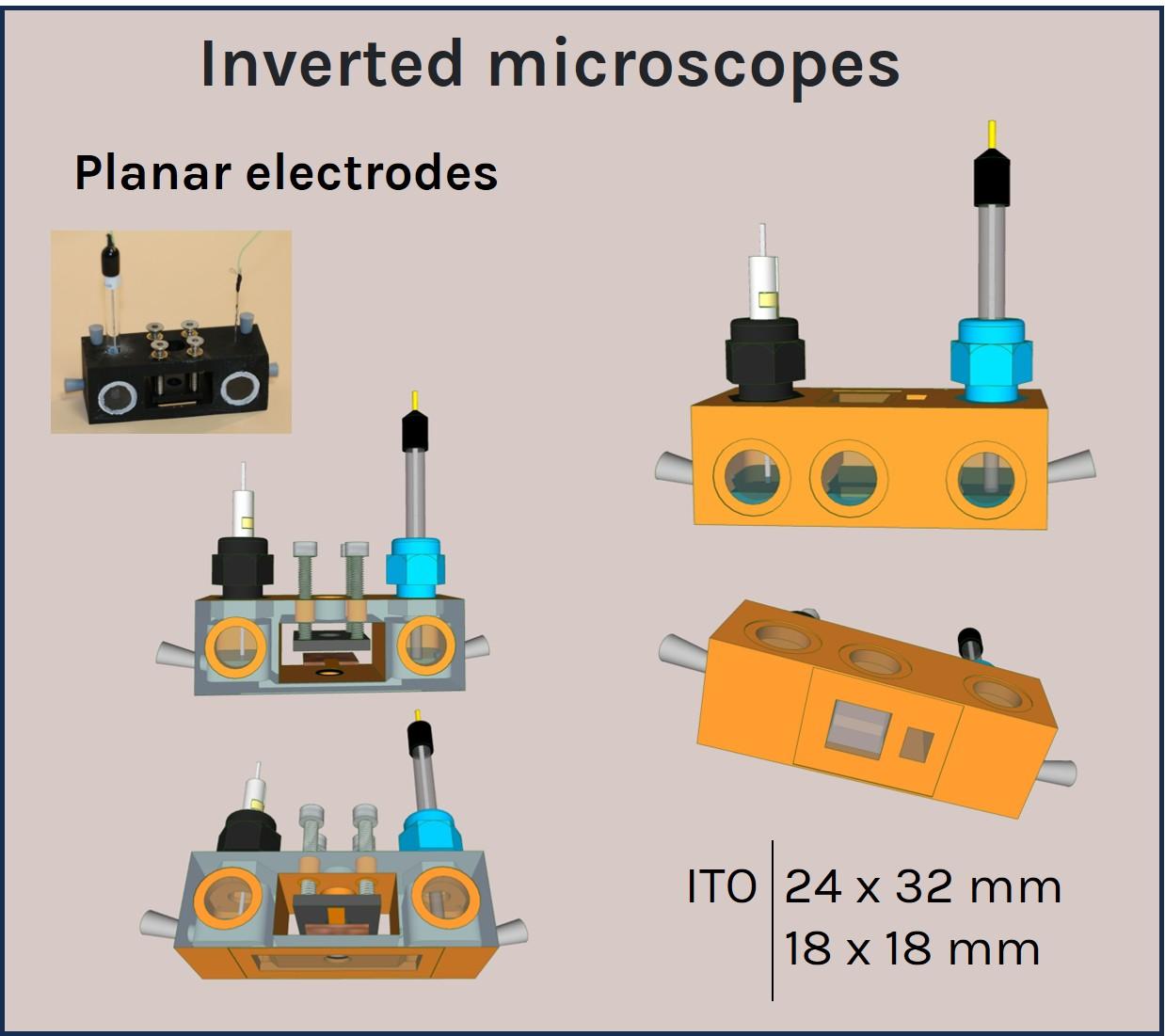
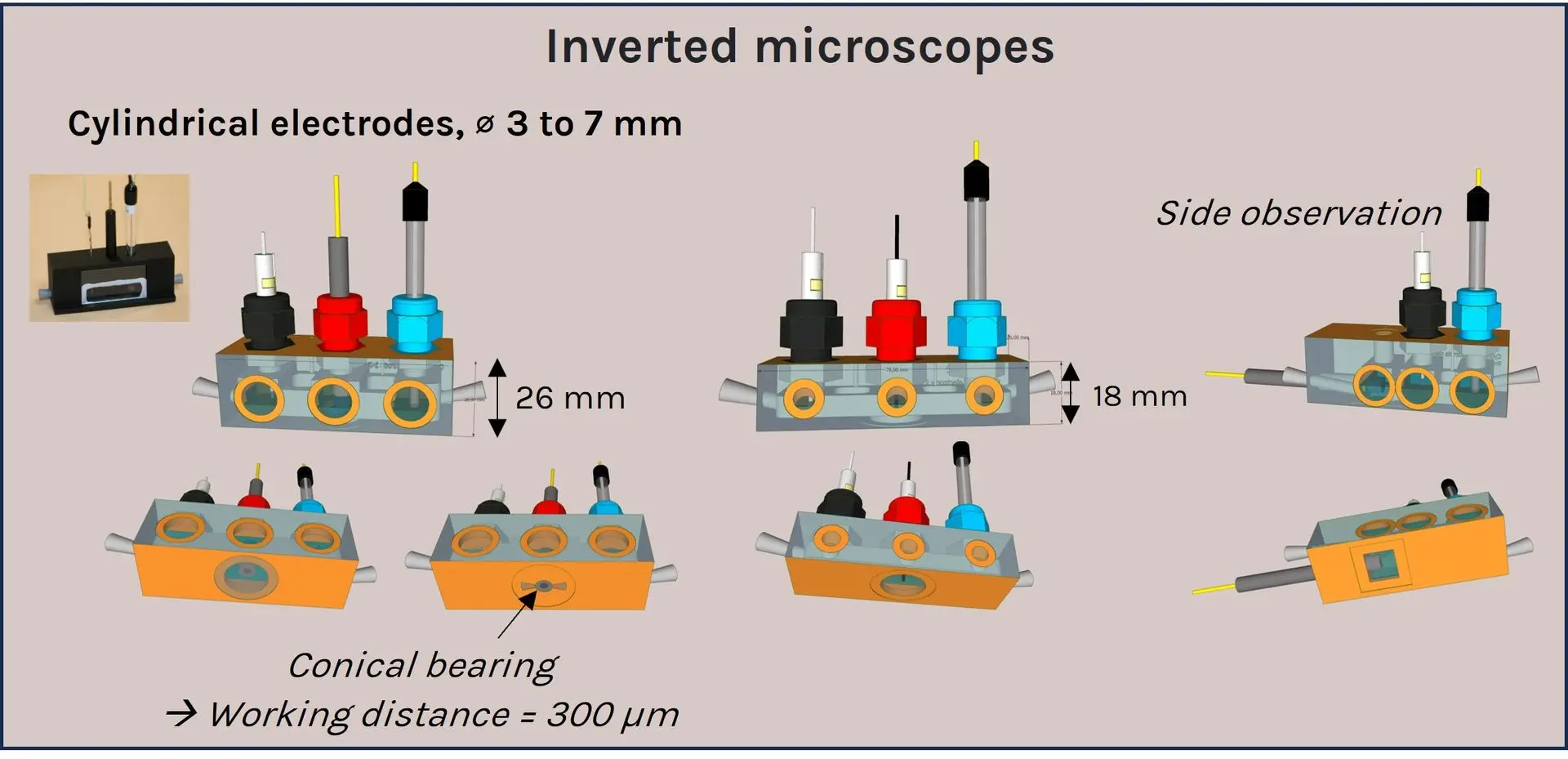
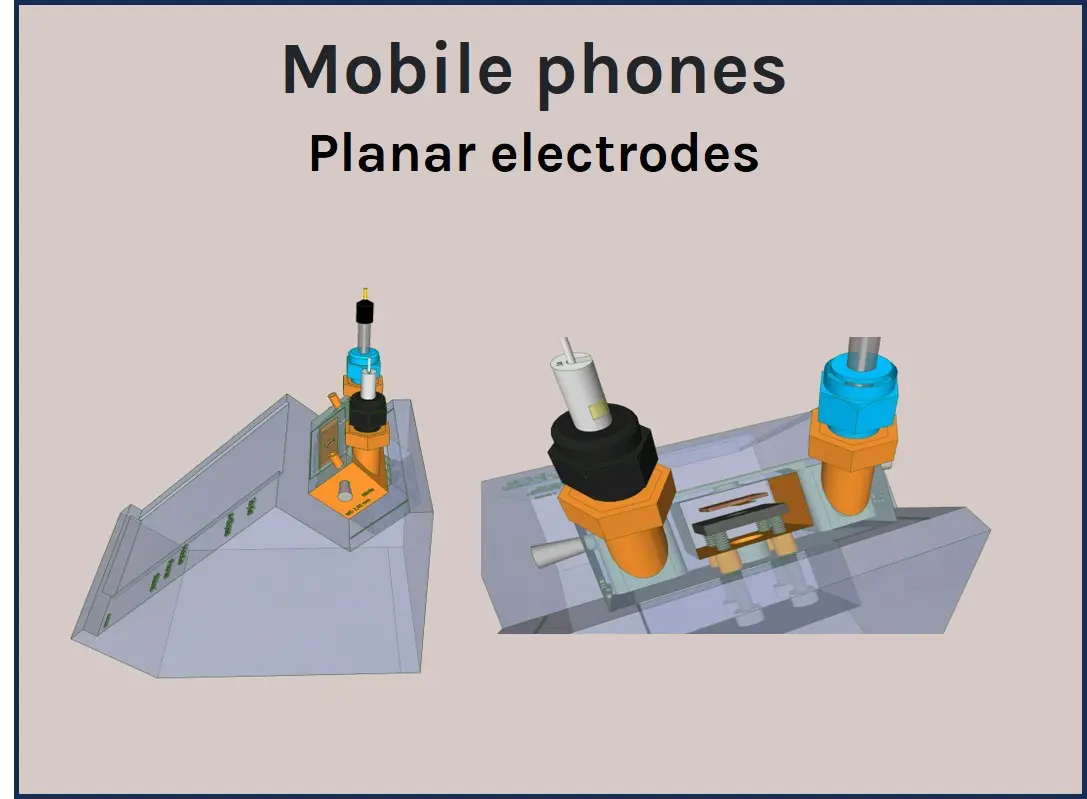
A panel of a-Clipse chambers, compatible with cylindrical and planar working electrode systems, is available to fit a variety of microscope and spectroscope instruments:
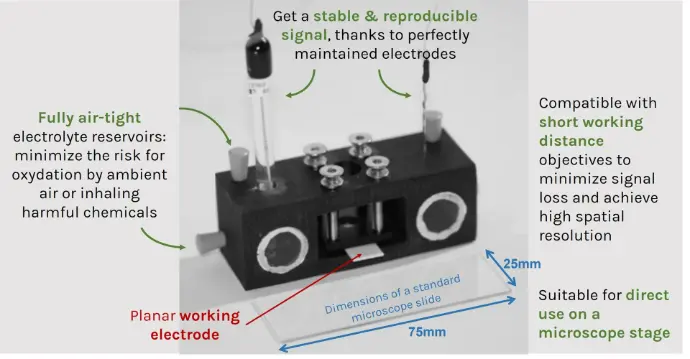
All a-Clipse chambers made for use with microscopes are made with dimensions of a standard microscope slide (25mm x 75mm) holder.
New ! None of them fulfills your experimental purposes? Contact us for on-demand electrochemical cells !
Kit contents
a-Clipse
chambers are provided with all small accessories that are required for their use. Electrodes are not included.
• Electrochemical cell
• Tapered plugs
• LUER connectors
• O-ring (for
a-CLIPSE P chambers only)
• Spare coverslips
Applications
Compatible assays (non-exhaustive list):
- Electrochemiluminescence microscopy (ECLM)
- Photoelectrochemistry
- Spectroelectrochemistry
a-Clipse
chambers can be used to image cells, micro-/nano-objects and electrochemical processes at electrode surfaces. So far,
a-Clipse
chambers have been mostly used in ECLM experiments but can be used for any assay aiming at visualizing electrochemical processes.
Some examples of ultra-sensitive ECLM applications
a-Clipse
chambers contributed to include:
- Imaging single cells & isolated subcellular organelles (i.e. cell membrane proteins & transport, cell-cell contacts, individual mitochondria imaging, etc)
- Development & optimization of (bio)analytical assays (i.e. map the spatial distribution of ECL emission at the surface of single micrometric beads)
- Investigating fundamentals of ECL (i.e. gain deeper insights into the ECL reaction & emission stability mechanisms)
Find out more details and example pictures in our Results section.
Additional resources
> Product overview
> Technical Datasheet
Any other questions? Please contact us.
Infrared Photoinduced Electrochemiluminescence Microscopy of Single Cells
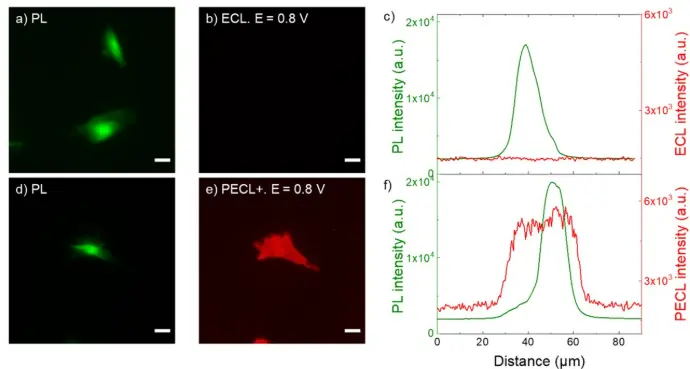
a,d) PL micrographs (green color) of CHO-K1 cells labeled with SA@Ru. b) ECL and e) PECL+ images (red color) of the corresponding labeled cells recorded in ProCell under near-infrared (λexc = 1050 nm) back-illumination at 0.8 V on b) p++-Si/SiOx/Ir and e) n-Si/SiOx/Ir electrodes. c,f) Comparison of the luminescence intensity profiles of the cells in PL, c) ECL and in f) PECL+. The axis along which the profiles were extracted are shown in Figure S5. Green and red are false colors coding the luminescence intensity. Cells were grown on the Ir surfaces, fixed, permeabilized with Triton X-100 and then labeled with SA@Ru. Scale bar: 20 µm. Images obtained with an a-Clipse P chamber for planar electrodes.
Credits: Julie Descamps et al, 2023
Using a-CLIPSE to develop a bimodal electrochemiluminescence imaging approach
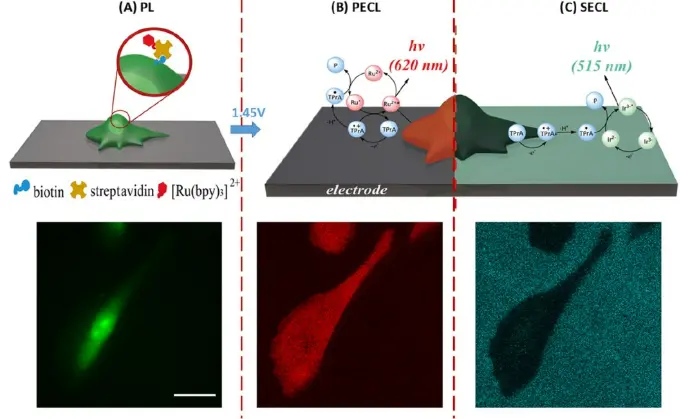
ECLIPSE chambers were used to demonstrate the potential of combining simultaneous positive (PL) and shadow ECL (PECL) configurations on single cells to gain insight into transport properties through permeabilized cell membranes. (Top) Schematic representation of multimodal imaging of a cell immobilized on a glassy carbon electrode (GCE): (A) PL, (B) PECL, and (C) SECL. Mechanisms of co-reactant PECL (heterogeneous route involving mainly dissolved TPrA and SA@Ru label immobilized on the cell) and SECL (homogeneous route involving only dissolved [Ir(sppy)3]3− and TPrA) modes. Ru2+ and Ir3− represent the ECL SA@Ru label and [Ir(sppy)3]3−, respectively. (Bottom) The same single CHO-K1 cell was imaged by (A) PL, (B) PECL, and (C) SECL. Images acquired in a-CLIPSE C chambers.
Credits: Sara Knezevic et al, 2023
Imaging plasma membranes at the single cell level using ECL microscopy
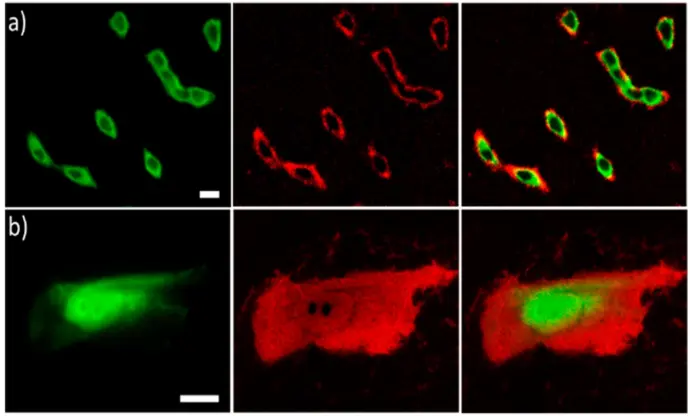
The ECLIPSE chambers were used to specifically image basal membrane details that are not resolved using classic fluorescence microscopy. PL (green), ECL (red) and overlay of both luminescence signals (from left to right) of CHO cells that were (a) labeled with SA@Ru or (b) permeabilized and then labeled with SA@Ru. Cells were grown on GC electrodes. SA@Ru labels were attached to the biotinylated proteins of the cellular membrane. Both PL and ECL images were recorded in the reflection configuration on the same regions of interest in the ECL focal plane. ECL was generated in PBS (pH = 7.4) containing 100 mM TPA by applying 1.4 V. Scale bar: 20 μm.
Images acquired in a-CLIPSE C chambers.
Credits: Silvia Voci et al, 2018
Individual mitochondria imaging in the a-CLIPSE chambers
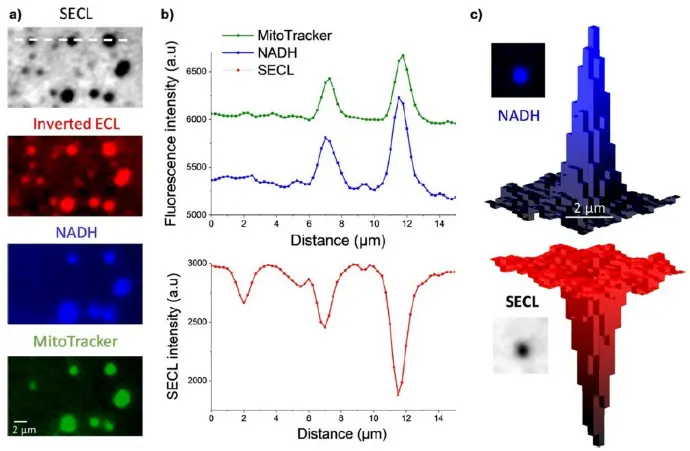
a) Typical SECL and fluorescence images recorded at high magnification of the same ROI showing single mitochondria. b) Luminescence intensity profiles extracted along the dashed line materialized on the top image in (a). c) An example of 3D imaging of a single mitochondrion recorded by NADH fluorescence (top) and SECL (bottom). Images acquired in a-CLIPSE C chambers.
Credits: Yumeng Ma et al, 2021
Using a-CLIPSE to investigate ECL electrochemical and photophysical properties on single micrometric decorated beads
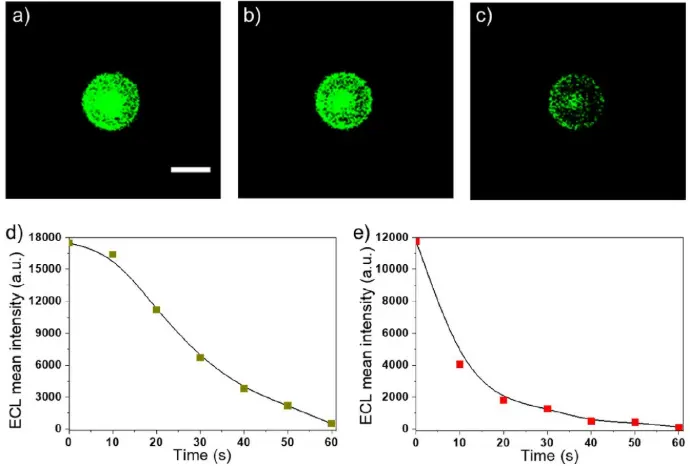
a-c) Sequence of successive ECL images of a single labeled bead recorded in the top-view configuration when imposing a constant potential of 1.1 V. PS 12 μm beads decorated with the ECL label were used. Exposure time: 10 s. Scale bar: 10 μm. Evolution of the ECL intensity with time on d) GC and e) gold electrode when applying a constant potential of 1.1 V. Experiments were performed with a GC or gold working electrode in a PBS solution containing 200 mM TPA (pH 7.4). Experiments have been repeated on more than 30 single beads under each set of conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Credits: Priyanka Dutta et al, 2020
Discover how to set up & use the a-Clipse chambers in videos:
Discover some groundbreaking studies featuring the a-CLIPSE chambers:
2024 | Infrared Photoinduced Electrochemiluminescence Microscopy of Single Cells
Julie Descamps, Yiran Zhao, Bertrand Goudeau, Dragan Manojlovic, Gabriel Loget, and Neso Sojic. Chem. Sci. 2024, https://doi.org/10.1039/D3SC05983A
2023 | Bimodal Electrochemiluminescence Microscopy of Single Cells
Sara Knežević, Emily Kerr, Bertrand Goudeau, Giovanni Valenti, Francesco Paolucci, Paul S. Francis, Frédéric Kanoufi, and Neso Sojic, Anal. Chem. 2023, 95, 7372−7378. https://doi.org/10.1021/acs.analchem.3c00869
2023 | Electrochemiluminescence Amplification in Bead-Based Assays Induced by a Freely Diffusing Iridium(III) Complex
Emily Kerr, Sara Knezevic, Paul S. Francis, Conor F. Hogan, Giovanni Valenti, Francesco Paolucci, Frédéric Kanoufi, and Neso Sojic, ACS Sens., 8, 933−939. https://doi.org/10.1021/acssensors.2c02697
2023 | Ultrasensitive Imaging of Cells and Sub-Cellular Entities by Electrochemiluminescence
Julie Descamps, Camille Colin, Gilles Tessier, Stéphane Arbault, and Neso Sojic, Angew. Chem. Int. Ed. 2023, 62, e202218574. doi.org/10.1002/anie.202218574
2022 | Enhanced electrochemiluminescence at microgel-functionalized beads
Dongni Han, Bertrand Goudeau, Véronique Lapeyre, Valérie Ravaine, Dechen Jiang, Danjun Fang, Neso Sojic, Biosensors and Bioelectronics 216, 114640. https://doi.org/10.1016/j.bios.2022.114640
2021 | Shadow Electrochemiluminescence Microscopy of Single Mitochondria
Yumeng Ma, Camille Colin, Julie Descamps, Stephane Arbault, and Neso Sojic. Angew. Chem. Int. Ed. 2021, 60, 18742–18749. doi.org/10.1002/anie.202105867
2021 | Electrochemiluminescence Microscopy of Cells: Essential Role of Surface Regeneration
Dongni Han, Bertrand Goudeau, Dechen Jiang, Danjun Fang, and Neso Sojic, Anal. Chem. 93, 1652-1657. https://doi.org/10.1021/acs.analchem.0c05123
2021 | Electrochemiluminescence Loss in Photobleaching
Dongni Han, Bertrand Goudeau, Dragan Manojlovic, Dechen Jiang, Danjun Fang, Neso Sojic, Angew Chem Int Ed Engl
. 2021 Mar 29;60(14):7686-7690. doi: 10.1002/anie.202015030
2020 | Reactivity mapping of luminescence in space: Insights into heterogeneous electrochemiluminescence bioassays
Priyanka Dutta, Dongni Han, Bertrand Goudeau, Dechen Jiang, Danjun Fang, and Neso Sojic, Biosensors and Bioelectronics 165, 112372. https://doi.org/10.1016/j.bios.2020.112372
2018 | Surface-Confined Electrochemiluminescence Microscopy of Cell Membranes
Silvia Voci, Bertrand Goudeau, Giovanni Valenti, Andreas Lesch, Milica Jović, Stefania Rapino, Francesco Paolucci, Stéphane Arbault, and Neso Sojic, J. Am. Chem. Soc. 2018, 140, 14753−14760. DOI: 10.1021/jacs.8b08080
2017 | Single Cell Electrochemiluminescence Imaging: From the Proof-of-Concept to Disposable Device-Based Analysis
Giovanni Valenti, Sabina Scarabino, Bertrand Goudeau, Andreas Lesch, Milica Jović, Elena Villani, Milica Sentic, Stefania Rapino, Stéphane Arbault, Francesco Paolucci, and Neso Sojic, J. Am. Chem. Soc. 2017, 139, 16830-16837. DOI: 10.1021/jacs.7b09260
The a-CLIPSE chambers are provided with all small accessories that are required for their use. All you need to have on your side is a set of working electrodes (cylindrical or planar), reference & counter electrodes, some sealing glue to replace the slides if needed and a M4 screwdriver (for a-Clipse P chambers only).
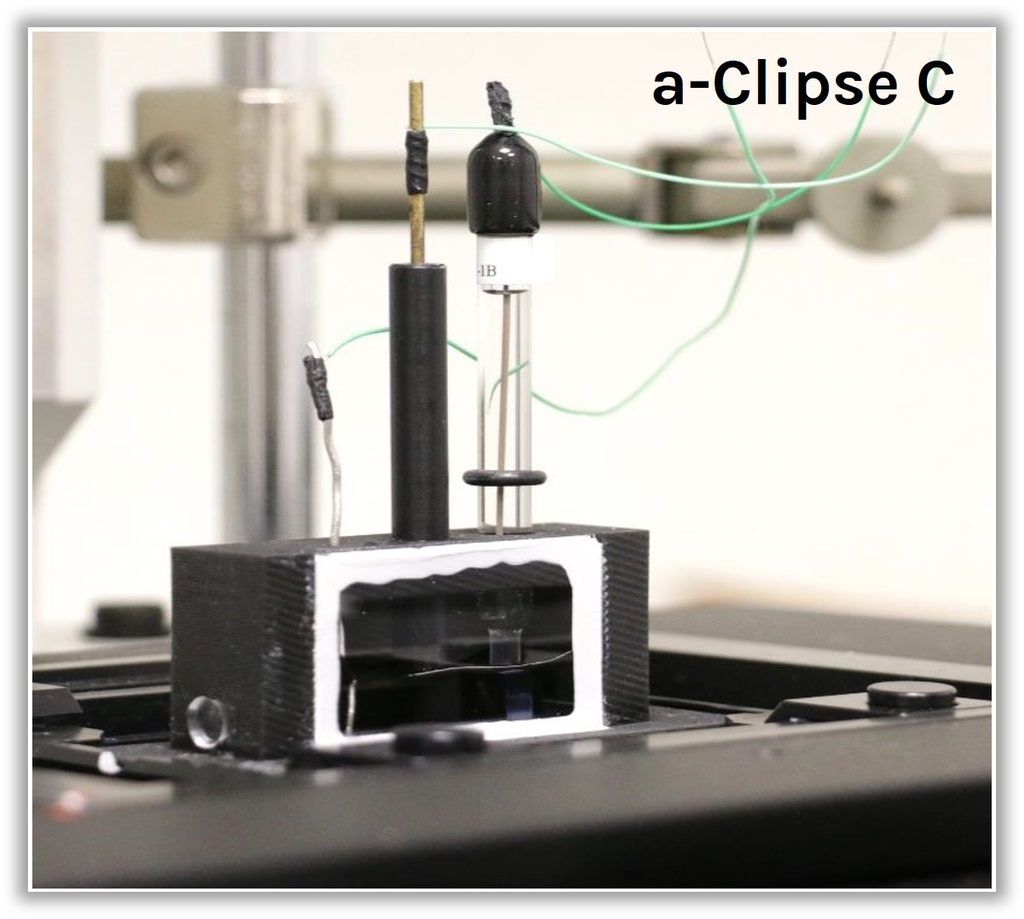
Two versions of the a-Clipse chambers are available for purchase, depending on your electrode geometry. The a-Clipse C chambers are compatible with standard cylindrical working electrodes, while a-Clipse P chambers are compatible with planar or screen-printed electrodes.
For a-Clipse C chambers, electrodes of other sizes can be adapted on the chambers using cable glands to adjust the opening diameter accordingly (typically down to ~3mm diameter).
For a-Clipse P chambers, the electrode needs to be large enough so that its surface creates a direct contact with the 7 mm o-ring placed below.
For the auxiliary electrode, any conductive and stable material can be used. In the demo video shown on our website, a 1 mm diameter platinum wire was used but a gold wire or a graphite rod of the same (or smaller) size could also work. Here is one example of a compatible reference (for 12.5cm of wire): https://www.goodfellow.com/fr/platinum-spooled-wire-1000165679
As for the reference electrode, also shown in that same video, a 6.2mm diameter Ag/AgCl electrode of the RE-1B type was used as described on this website: https://www.als-japan.com/1388.html
There are many different diameters and types available and several suppliers offer them. Here are a few other options: www.palmsens.com/products/sensors/classic-electrodes/collection/electrode-type-reference-electrode/
Total filling volumes are 4.4 mL for the type C cell and 12.9 mL for the type P cell. Note that these are maximal filling volumes: lower volumes can be loaded according to the specific experimental goals and constraints.
The a-CLIPSE chambers can be placed on any inverted microscope equipped with a standard microscope slide (25mmx75mm) holder. A specific chamber for upright microscopes tilted to 90 degrees is available on request. Contact us if interested.
Yes, cells can be plated in adhesion to the electrodes by using a specific setup and imaged in the a-CLIPSE chambers after fixation. Alternatively, suspension of isolated organelles can be deposited on an electrode and visualized by ECL microscopy in the a-CLIPSE chambers.
The a-Clipse chambers can easily be used for electrolytes sensitive to water and oxygen, as the compartments can be completely sealed: potential leak areas are located where the glass slides are fixed, as well as at the openings cut in the plastic (made for inserting the electrodes or cleaning the electrolyte reservoirs). For all these sources, it is possible to guarantee completely airtight conditions: as for the glass slides, they can be bonded with a silicone adhesive that is impermeable to oxygen (we can provide this with the chambers). For the openings, we provide rubber conical caps or Luer connector-type fittings depending on the needs.
The port sizes on electrolyte reservoirs are perfectly adjusted to electrode dimensions, therefore greatly minimizing the risk for harmful chemical inhalation. If needed, PTFE (Teflon) tape can be used to help adjust electrodes and ensure you work in fully air-tight conditions.
a-CLIPSE C chambers are compatible with high magnification and short working distance (300µm) objectives. Examples of objectives used with a-CLIPSE C chambers include the HC PL APO 63X objective, aperture = 1.3, free working distance = 300µm (Leica, Cat#11506398). You may need to polish the electrode edges to get your desired working distance.
Allowing imaging at a working distance of 2.95mm, we recommend using
a-CLIPSE P
chambers
with objectives
up to 40X.
Examples of objectives used with
a-CLIPSE P chambers include the HC APO 40X objective, aperture = 0.8, free working distance = 3.3mm (Leica, Cat#11506155).
Yes, optical fibers or photomultipliers can be placed underneath EC chambers for spectroscopic measurements. The main thing to keep in mind is to find a fixed position to ensure good stability and reproducibility across experiments.
To further optimize your spectroscopy workflow, another chamber design dedicated to spectroscopic measurements is available on request. Please contact us if interested.
Yes, the a-Clipse chambers can be connected to Luer Lock connectors to work in dynamic flow conditions.
Yes, both the upper part of the chamber and the plastic part placed on the top of the planar electrode contain a circular opening, so it is entirely possible to illuminate the back of the electrode and to observe its surface (covered with metal) from below.
Check out our Publications section for example results of cells imaged with back-illumination configuration in a P-type chamber.
Conventional solvents can be flowed inside the a-Clipse chambers for cleaning (water, ethanol). If using organic solvents (ie acetonitrile), the sealing glue should be replaced with a compatible one (i.e. Wacker Elastosil E43).
These chambers have certainly been widely used by inventors for photoluminescence, but they can also be perfectly suited for any experiment aiming to combine electrochemistry and a light source. In fact, the design of these chambers was primarily conceived to meet a number of criteria that can be useful in various applications:
- Optical accessibility, thanks to the integrated windows on both types of chambers
- Configuration of the working, reference, and counter electrodes, inserted into ports specifically adjusted according to their dimensions and ensuring their stability
- A material selected for its compatibility with electrolyte solutions, and perfectly sealed reservoirs to guarantee user safety and prevent contamination by ambient gases and humidity
- Compatibility with dynamic experiments, thanks to the side openings compatible with 'Luer Lock' connectors
The resin making up the chambers has been selected to guarantee full compatibility with standard solvents used in electrochemistry applications without conducing electric current. The chambers have been used with acetonitrile and KOH 0.1 M without any issues.
The default windows provided with the a-Clipse cells are coverglasses made of pure white Hydrolytic Class 1 glass with a thickness of 0.13 - 0.16 mm. That being said, it is perfectly possible to replace the windows located on the lateral and/or bottom sides of the chambers if needed (i.e. quartz, calcium fluoride, etc). To do this, you simply have to remove them carefully and glue new coverslips with silicone paste. The needle provided in the kit can be used on top of a syringe to facilitate paste spreading. Some examples of silicon pastes you can use are Elkem CAF 4 or Wacker Elastosil E43.
Yes, the a-Clipse chambers are a well-established method that had been successfully used worldwide in a variety of published studies since 2017. A full list of publications is available in the "Publications" section.
Facing the lack of standard devices to directly couple electrochemical reactions with microscopic or spectroscopic readouts, these ready-to-use chambers have rapidly become a pivotal feature in workflows where real-time monitoring of electrochemical processes is key. Indeed, these enclosed cells allow experimental conditions to be precisely controlled and maintained, minimizing sample disturbance and ensuring reliable and reproducible data: this is usually why scientists tend to turn to these a-Clipse cells, which also have the advantage of saving a great amount of time and hassle compared to home-made systems that often need to be developed and characterized from scratch.
Any other questions? Please contact us.