- All Products
- CrystalChip - Microfluidic chips for protein crystallization
CrystalChip - Microfluidic chips for protein crystallization
https://www.idylle-labs.com/shop/crystalchip-microfluidic-chips-for-protein-crystallization-492 https://www.idylle-labs.com/web/image/product.template/492/image_1920?unique=fbb9a9eScreen thousands of conditions at once, with only a small amount of protein sample
A technology developed by Claude Sauter (Institut de Biologie Moléculaire et Cellulaire IBMC) - Strasbourg, France
Why should I use CrystalChip ?
CrystalChip provides a single tool for all your crystallization experiments:
- Screening of crystallization solutions: CrystalChip offers as an alternative to vapor diffusion methods for a simpler and cost-effective routine screening of crystallization conditions. Instead of testing hundreds of individual conditions, CrystalChip allows the formation of continuous concentration gradients to make sure you never miss out the nucleation point. With only 5 chips and 25 µL of purified protein, create the 40 precipitant gradients that were shown to successfully crystallize 100% of tested proteins [1]. CrystalChip also provides a perfect system to optimize and refine pre-selected crystallization conditions in a convection-free environment.
- Determination of crystal structure: CrystalChip design has been optimized to perform in situ characterization by X-ray diffraction. The microfluidic format promotes the formation of multiple randomly oriented micro-crystals along the channels, homogeneous in size and quality, that you can directly bring into the X-ray beam to reconstitute a complete dataset and solve the 3D structure. Unlike traditional data collection performed on single crystals at cryogenic temperature, this serial approach carried out at room temperature allows to best preserve the crystal diffraction properties, reflected by a low mosaicity. It means there is no need to manually fish out crystals, avoiding potentially deleterious manipulation. And finally, you can access real-time monitoring of dynamic processes within crystals, opening exciting perspectives in structural biology!
- Crystal imaging: thanks to its transparent material and standard microscope slide format, the chip can be directly placed on a microscope stage or in a crystal imager to monitor crystal formation, protein-ligand interactions or to select the best candidates for structural studies or cryo-EM workflows. Once formed, all crystals are on a same focal plane with no risk of evaporation or contamination. Imaging crystals has never been easier!
Join us in this webinar to learn more about how combining the advantages of the microfluidic format with high-throughput imaging opens exciting perspectives in protein crystallization studies!
How does CrystalChip work ?
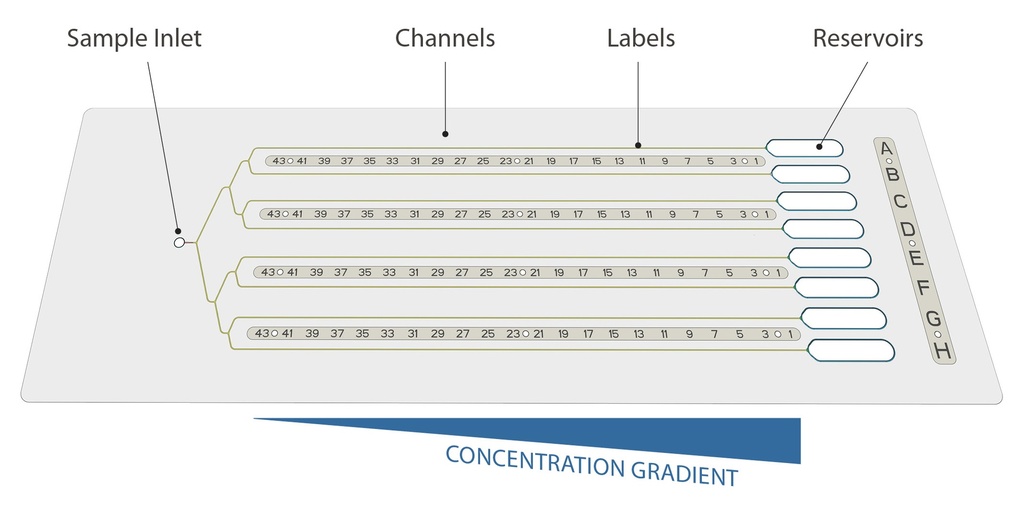
CrystalChip is composed of a single injection port to load 5 µL of purified protein, connected to 8 separate microfluidic channels in which individual concentrated crystallizing solutions are loaded. Once injected, crystallizing agents immediately diffuse into the channels and create concentration gradients evolving dynamically, a very favorable convection-free environment to trigger nucleation & growth of high-quality crystals.
From the beginning or after crystal growth, external compounds (i.e. protein ligands, enzyme substrates, compounds for fragment screening, and anomalous scatterers for phasing) can be added to the reservoir solutions to be incorporated in the crystals. Once formed, crystals can be safely stored and transported for months.
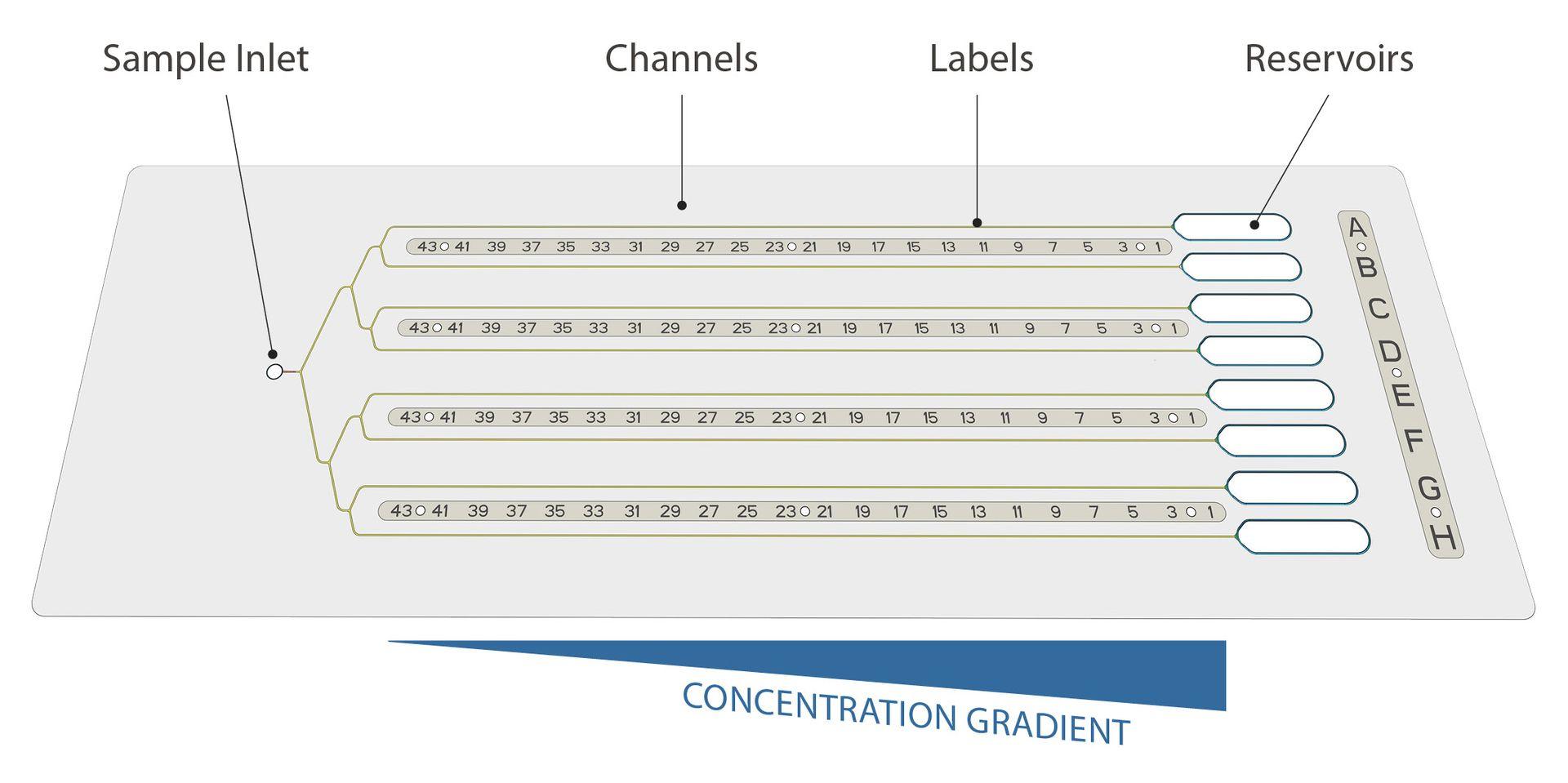
Use less, get more: thanks to its 4.5 cm long channels, CrystalChip allows you to explore a continuum of supersaturation states and is highly efficient at screening potential nucleation conditions while using minimal sample amounts. With only 5 chips, or 25 µL of purified protein, you can quickly try out 40 crystallizing cocktails at infinite concentrations instead of testing hundreds of individual conditions using standard vapour diffusion or batch methods.
Simplify your biocrystallography workflow: CrystalChip can be easily positioned on goniometers and translated in the X-ray beam, allowing for direct analyses of crystals inside the chip at room temperature and without handling.
Access more physiological conditions: unlike traditional data collection performed on single crystals rotating in the X-ray beam at cryogenic temperatures, a complete diffraction space is reconstructed from a series of randomly oriented crystals analyzed at room temperature along the channels. This has the advantage of preserving physiological crystal quality and diffraction properties, ensuring low mosaicity. It also avoids crystal damage due to handling and allows real-time monitoring of dynamic processes within crystals such as enzymatic catalysis.
Proteins already crystallized with CrystalChip:
CrystalChip has been successfully used to crystallize various soluble & membrane proteins (enzymes alone and in complex with substrates, nanobodies, proteases, lipases, tRNA synthetases, hemoglobin) and an RNA duplex. Find out detailed examples of CrystalChip uses in the Results & Publications sections.
Discover more on CrystalChip & the counter-diffusion method by hearing directly from its inventor:

Applications of CrystalChip
- Biomacromolecule crystallization: use it as a routine screening tool for finding the right crystallization conditions, refine your conditions after an initial screening or increase your chance of success when working with recalcitrant proteins.
- On-chip treatments: external compounds can be loaded in the channels throughout the crystallisation process (i.e. in situ crystal soaking with ligands) for substrate catalysis, ligand screening in fragment-based drug design or phasing purposes.
- Crystal imaging: using standard microscopy & spectroscopy methods (phase contrast, UV-illumination, fluorescence imaging, polarized light, etc). A useful method to select best candidates for structural studies or investigate fundamentals of crystal nucleation and growth processes in different conditions, and a smart tool for educational and demonstration purposes!
- In situ structural analysis by serial crystallography: thanks to a dedicated holder, the chip can be directly positioned on synchrotron beamlines or XFELs for structural characterization using serial protocols at room temperature. Partial datasets collected on series of crystals formed along the channels are compared, sorted and then combined to lead to the determination of the 3D structure of the crystallized biomolecule.
- Crystal storage, handling & shipment: as all steps are performed in situ without handling, crystals can be safely stored for months or transported in the chip while preserving their intrinsic quality.
CrystalChip can provide a straight-forward complementary tool to use alongside single particle cryo-EM if you need to maximize resolution and detail, resolve small or highly dynamic structures that may present challenges for cryo-EM, boost your data collection speed or simply to address different aspects of the sample.
CrystalChip can directly be mounted on Synchrotron beamlines
Storage: Unused chips can be stored indefinitely when protected from light.
[1] Efficient Screening Methodology for Protein Crystallization Based on the Counter-Diffusion Technique. Luis A. González-Ramírez, Carlos R. Ruiz-Martínez, Rafael A. Estremera-Andújar, Carlos A. Nieves-Marrero, Alfonso García-Caballero, José A. Gavira, Juan López-Garriga, and Juan M. García-Ruiz. Crystal Growth & Design 2017 17 (12), 6780-6786. DOI: 10.1021/acs.cgd.7b01353
This miniaturized device, with the size of a microscope slide, integrates all steps from biomacromolecule crystallization to high-resolution structural analysis: crystals are produced by counter-diffusion, can be directly visualized, soaked with ligands and analyzed in situ for crystal structure determination.
CrystalChip aims at facilitating access to serial approaches to make other scientists benefit from it.
CrystalChip at a glance
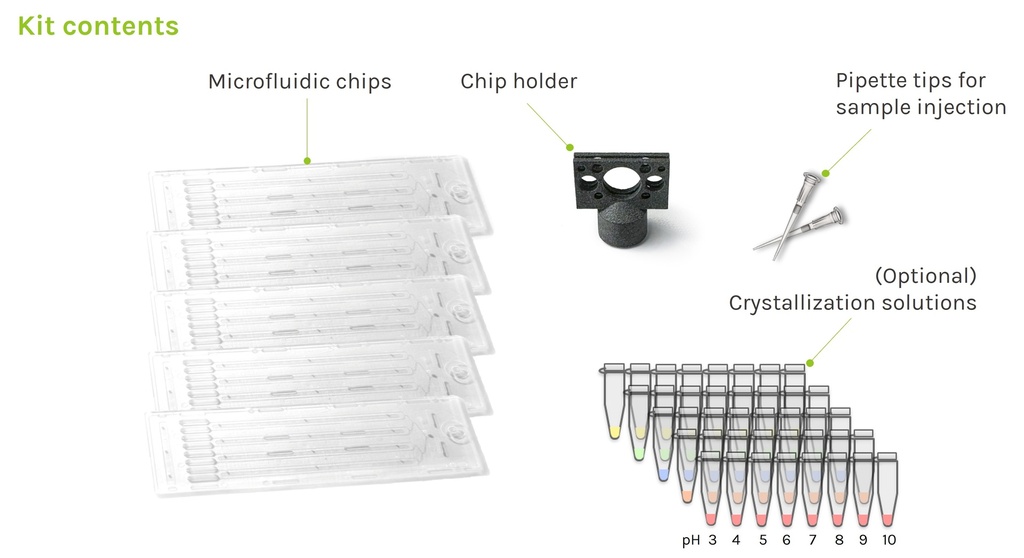
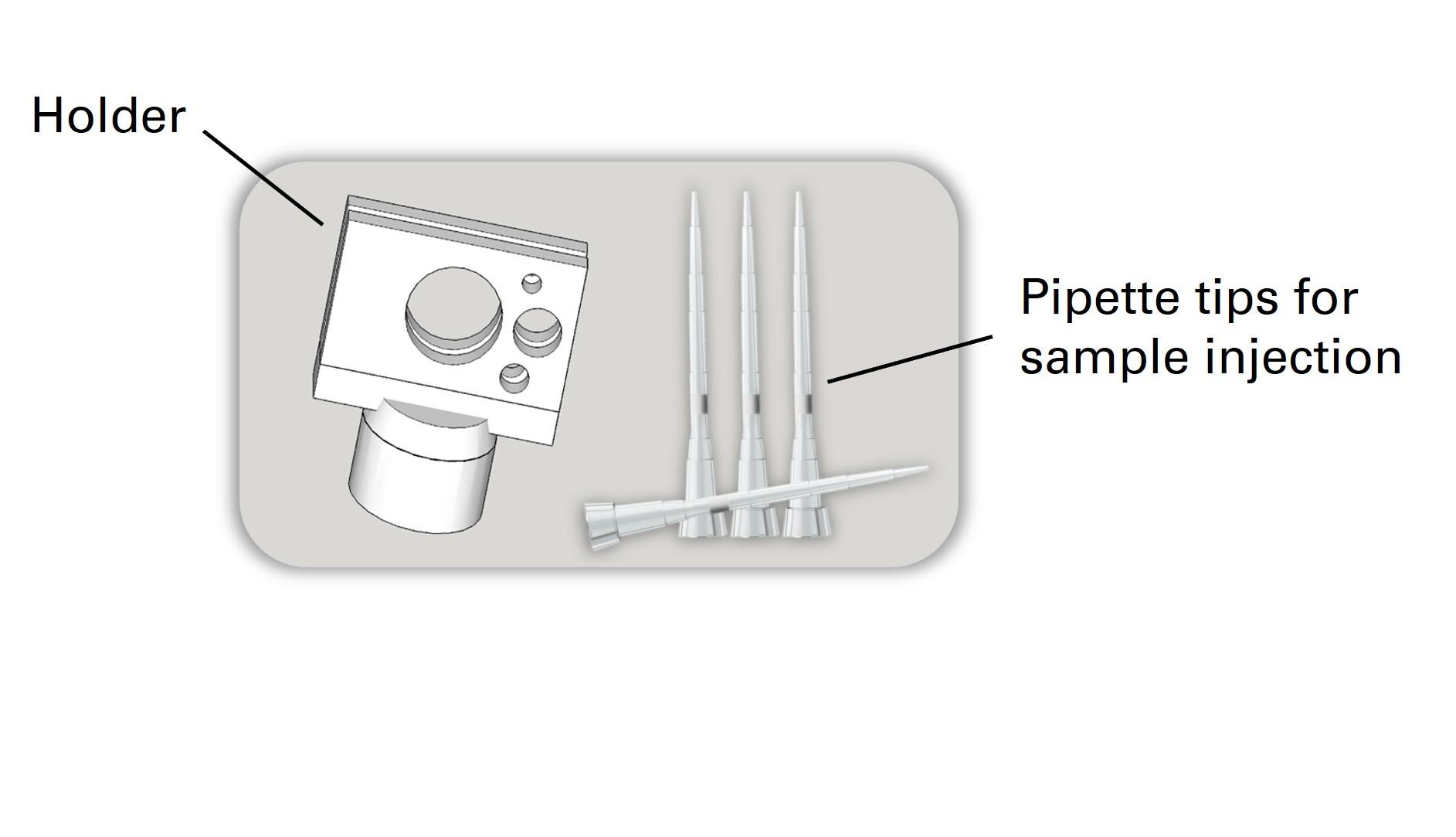
Kit contents of CrystalChip:
Whether you want to use CrystalChip for routine screening of crystallization conditions, or as a complementary tool to optimize your conditions, add ligands or perform in situ analyses, we provide various kit formats to fit your purposes.
Discovery kit
- 5x microfluidic chips
- 10x 10µL pipette tips for sample injection
- 1x chip holder
Discovery screening kit
- 5x microfluidic chips
- 10x 10µL pipette tips for sample injection
- 1x chip holder
- 40x ready-to-use crystallizing mixes
Extended kit
- 30x microfluidic chips
- 60x 10µL pipette tips for sample injection
- 1x chip holder
Extended screening kit
- 30x microfluidic chips
- 60x 10µL pipette tips for sample injection
- 1x chip holder
- 40x ready-to-use crystallizing mixes
All kit options include some 10 µL pipette tips specifically adapted to the sample injection port, as well as a holder for direct chip mounting on goniometers using standard magnetic heads.
CrystalChip screening kits
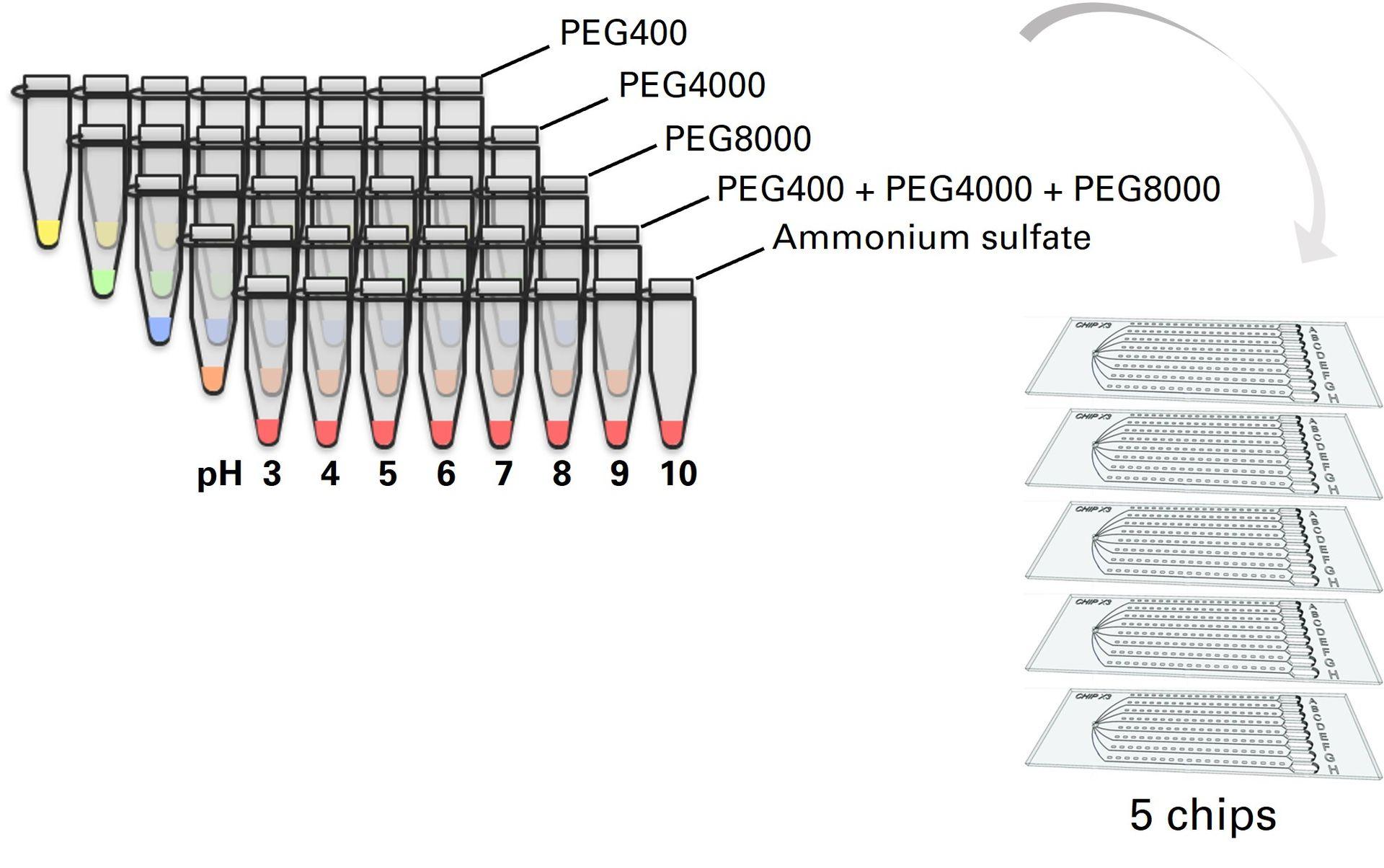
In addition to the basic kit contents, screening kits also include 40 ready-to-use crystallization solutions to perform highly-efficient screening of crystallization conditions for a wide range of proteins with only 5 chips. These crystallizing cocktails are composed of concentrated PEG & ammonium sulphate solutions with pH ranging from 3 to 10. They were specifically optimized for use with the counter-diffusion method, and were shown to successfully crystallize 100% of tested proteins [1]. We strongly recommend using these when performing initial screening of crystallization conditions.
[1] Efficient Screening Methodology for Protein Crystallization Based on the Counter-Diffusion Technique. Luis A. González-Ramírez, Carlos R. Ruiz-Martínez, Rafael A. Estremera-Andújar, Carlos A. Nieves-Marrero, Alfonso García-Caballero, José A. Gavira, Juan López-Garriga, and Juan M. García-Ruiz. Crystal Growth & Design 2017 17 (12), 6780-6786. DOI: 10.1021/acs.cgd.7b01353
Examples of crystals obtained in CrystalChip
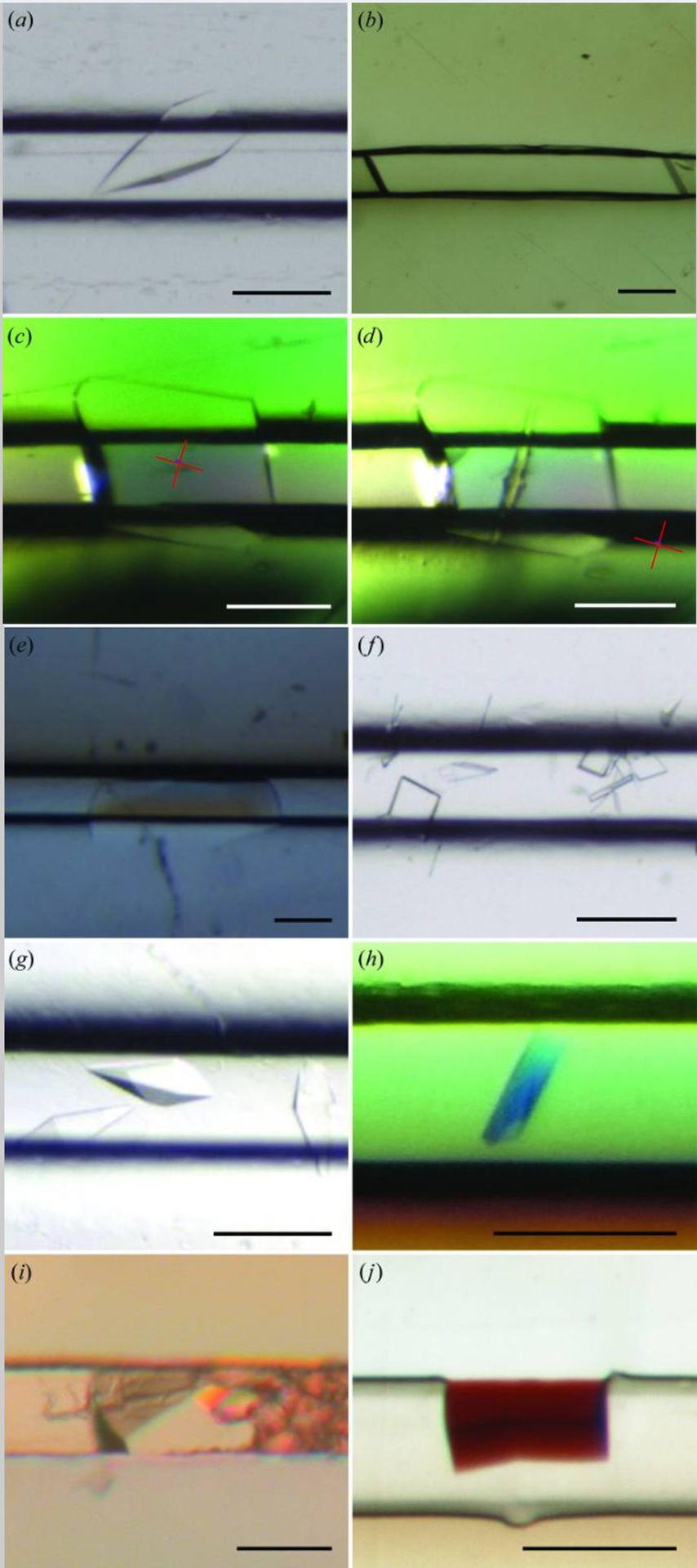
Credits: de Wijn R, Hennig O, Roche J, Engilberge S, Rollet K, Fernandez-Millan P, Brillet K, Betat H, Mörl M, Roussel A, Girard E, Mueller-Dieckmann C, Fox GC, Olieric V, Gavira JA, Lorber B, Sauter C. A simple and versatile microfluidic device for efficient biomacromolecule crystallization and structural analysis by serial crystallography. IUCrJ. 2019 Apr 19;6(Pt 3):454-464. doi: 10.1107/S2052252519003622. PMID: 31098026; PMCID: PMC6503916.
Studying local conformational adaptations upon enzyme substrate binding using CrystalChip
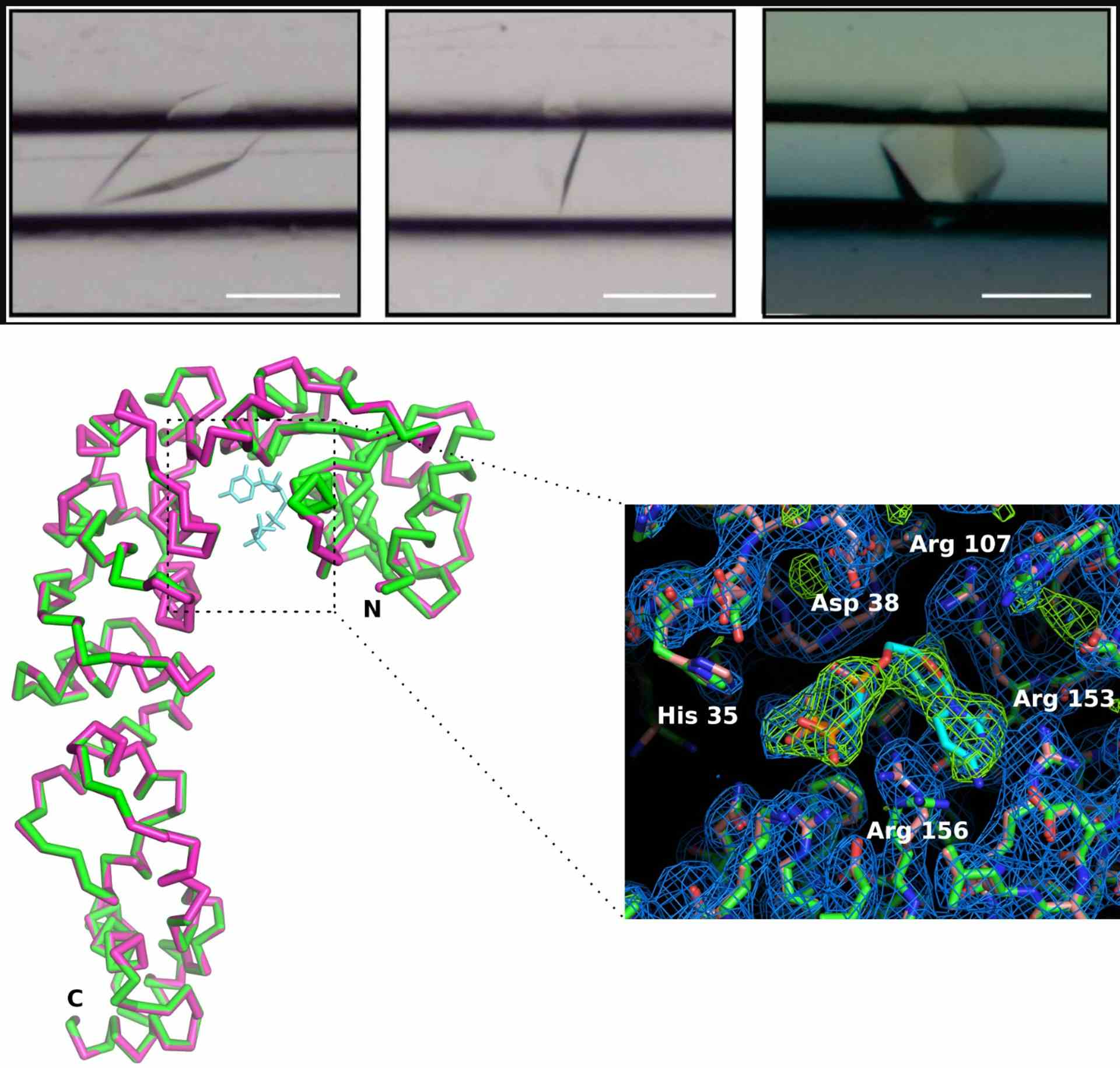
Credits: de Wijn R, Rollet K, Olieric V, Hennig O, Thome N, Noûs C, Paulus C, Lorber B, Betat H, Mörl M, Sauter C. Crystallization and Structural Determination of an Enzyme:Substrate Complex by Serial Crystallography in a Versatile Microfluidic Chip. J Vis Exp. 2021 Mar 20;(169). doi: 10.3791/61972. PMID: 33818565.
Crystal detection in CrystalChip by fluorescence
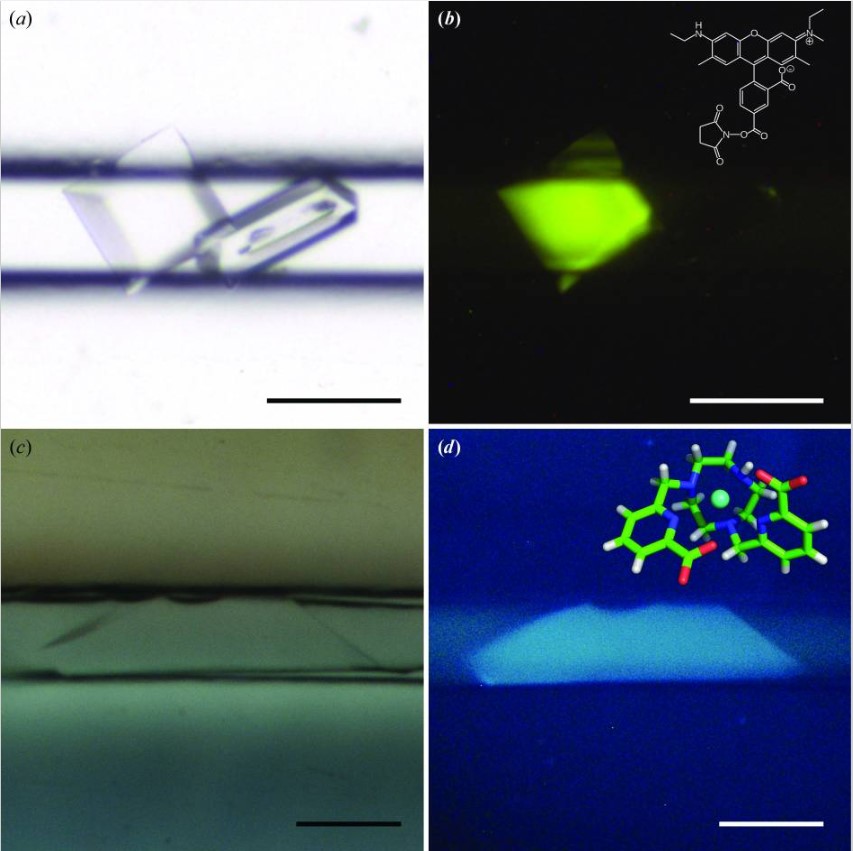
Credits: de Wijn R, Hennig O, Roche J, Engilberge S, Rollet K, Fernandez-Millan P, Brillet K, Betat H, Mörl M, Roussel A, Girard E, Mueller-Dieckmann C, Fox GC, Olieric V, Gavira JA, Lorber B, Sauter C. A simple and versatile microfluidic device for efficient biomacromolecule crystallization and structural analysis by serial crystallography. IUCrJ. 2019 Apr 19;6(Pt 3):454-464. doi: 10.1107/S2052252519003622. PMID: 31098026; PMCID: PMC6503916.
How to use CrystalChip in pictures
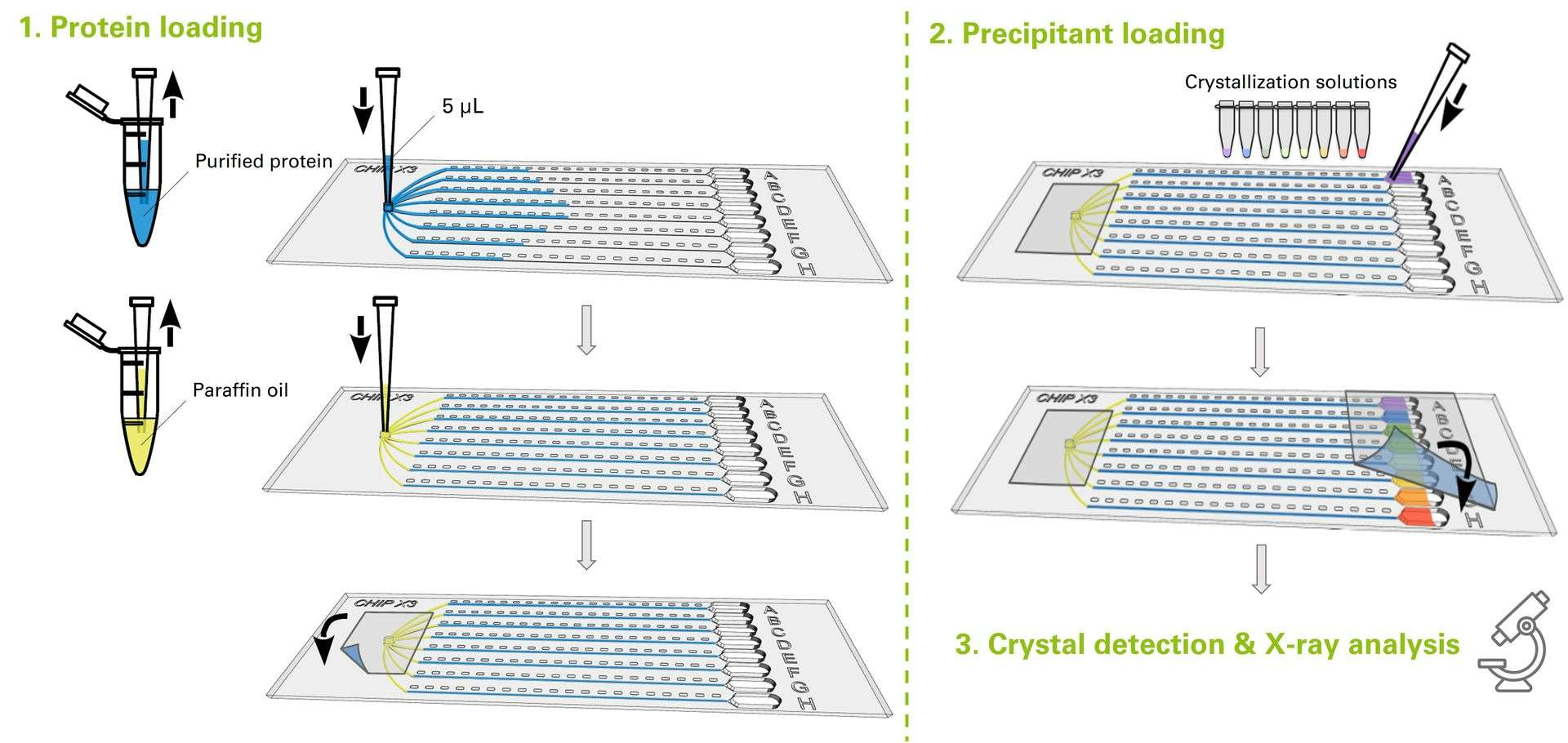
The counter-diffusion method consists in directly mixing the sample and precipitant solutions by putting them in contact in a restricted geometry. As soon as the sample and precipitant solution are loaded, a continuous gradient of concentrations is created by liquid-liquid diffusion. This system allows to explore a wide range of supersaturation conditions in a single experiment while keeping sample volumes to a minimum.
Although the counter-diffusion method has already proven its worth as an excellent choice for screening purposes, its widespread adoption has been so far restricted by experimental setup complexity, limited access to sophisticated equipments and costly microfabrication techniques.
CrystalChip makes counter-diffusion directly available to researchers by offering a user-friendly & standardized screening tool. In addition, its microfluidics design implemented on a standard microscope slide format unlocks potential to add ligands, directly visualize crystal growth, determine crystal structure in situ without handling and store high-quality crystals on the long-term, opening exciting perspectives in crystallography!
No, we do not recommend using CrystalChip in cryogenic conditions. As multiple microcrystals will form along each channel, the CrystalChip design is fully optimized for serial crystallization collection at room temperature, which has the advantage of operating at more physiological temperatures and does not require any handling of the crystals. If using a XFEL, each crystal introduced into the beam will produce a single image before being destroyed. If using a synchrotron with a beam less intense, a partial dataset is usually collected on each crystal before radiation damage, thus reducing the number of samples required to determine a structure.
Unlike traditional data collection performed on single crystal rotating in the X-ray beam at cryogenic temperature, a series of randomly oriented crystals is analyzed at room temperature to reconstruct a complete diffraction space. Serial crystallography has the advantage of operating at more physiological temperatures and allows crystals to reveal their full diffraction potential after characterized by a very low mosaicity. Serial crystallography also unlocks real-time monitoring of dynamic processes within crystals, such as enzymatic catalysis, opening a new field of investigation in structural biology with the possibility of producing real molecular films.
Precise control and reproducibility of crystallization conditions are essential in serial crystallography, as the process depends on producing multiple crystals of consistent size and quality, often with limited biomolecule samples. The CrystalChip’s microfluidic design supports extreme miniaturization and high-throughput serial crystallization experiments, offering a convection-free environment that promotes the growth of high-quality crystals. Additionally, it provides an efficient means of positioning crystals in the X-ray beam for serial diffraction experiments.
CrystalChip has been successfully used to crystallize various soluble & membrane proteins, alone or in complex with substrates. Successful crystals have been obtained with the following biomolecules (non-exhaustive list):
- CCA-adding enzyme
- lysozyme
- thaumatin
- insulin
- nanobody from llama
- protease
- lipase
- tRNA synthetases (human & bacterial)
- membrane transporters
- hemoglobin
- RNA duplex
Find out example results and more detailed information on buffer, crystallant solutions used & resolutions achieved for each protein in the Results & Publications sections.
Total duration for crystal formation can vary greatly depending on each individual biomolecule characteristics. Some proteins will crystallize in a few minutes (i.e. lysozyme) up to a few days or weeks. Chips are typically checked under the microscope over a period of 2-4 weeks to track the growth of crystals.
CrystalChip is a user-friendly tool that does not require any sophisticated equipment. We provide you with microfluidic chips, accurate pipet tips for sample loading and a holder to position your chip on synchrotron or XFEL facilities. In option, you can add ready-to-use crystallization solutions to your order. All you need to have on your end is your purified protein and, if needed, some paraffin oil.
We strongly recommend using the provided crystallization solutions when using CrystalChip for initial screening of conditions. These crystallization cocktails, made of the most successful precipitating agents typically employed in protein crystallization, were specifically optimized for the counter-diffusion method and were shown to crystallize all samples in a previous study testing 14 different proteins [1].
Alternatively, you can load your own precipitating solution and use CrystalChip as an optimization tool, treat your crystals with ligands or analyse them in situ.
We do not recommend using standard commercial kits for initial screening with CrystalChip. The counter-diffusion method will work best with precipitating agents concentrated up to the maximum of their solubility at 4°C, which is not typically the case with commercial kits designed for vapour diffusion or batch methods. If initial crystallization conditions were obtained by vapor diffusion, the crystallant concentration should be increased by a factor of 1.5-2.
[1] Efficient Screening Methodology for Protein Crystallization Based on the Counter-Diffusion Technique. Luis A. González-Ramírez, Carlos R. Ruiz-Martínez, Rafael A. Estremera-Andújar, Carlos A. Nieves-Marrero, Alfonso García-Caballero, José A. Gavira, Juan López-Garriga, and Juan M. García-Ruiz. Crystal Growth & Design 2017 17 (12), 6780-6786. DOI: 10.1021/acs.cgd.7b01353
You can label your protein with a fluorescent compound to facilitate crystal identification and their discrimination from salt crystals.
Crystals cannot be extracted from their microfluidic channels once formed in CrystalChip. Yet, the CrystalChip method provides a fully integrated pipeline for the determination of crystal structure, in which all steps are carried out in situ at room temperature. Therefore, there is no need to retrieve the crystals or perform the diffraction analysis at cryogenic conditions. This avoids altering crystal mosaicity by physical interaction or cryocooling, preserving their genuine diffraction properties.
All our CrystalChip kits include a 3D-printed chip holder, that was specifically designed to attach electromagnets present on standard goniometers. This holder has been notably used for X-ray analysis at diverse synchrotron facilities across Europe (SOLEIL & ESRF, France and SLS, Switzerland).
Alternatively, it is also possible to load the chip in a universal frame (i.e. microplate format) if a robotic arm is used, or if using the chip in an imager. Chip dimensions are that of a standard microscope slide (25mmx75mm), so it can be placed in any commercially available frame that will fit that format. Please contact us at [email protected] for further advice.
The CrystalChip has been used for X-ray analysis at diverse synchrotron facilities across Europe, and diffraction data were collected on the following beamlines:
- Swiss Light Source (SLS), Villigen, Switzerland: beamline PXII equipped with a PILATUS 6M detector & beamline PXIII equipped with a MAR CCD or a PILATUS 2M-F detector, X10SA and X06DA beamlines equipped with a MAR MX225 CCD detector or DECTRIS PILATUS 2M/6M pixel detectors.
- SOLEIL, Saint-Aubin, France: PROXIMA-2A (PX2A) beamline equipped with an EIGER X 9M detector
- ESRF, Grenoble, France: beamline ID30B equipped with a PILATUS3 6M detector, FIP-BM30A beamline equipped with an ADSC Quantum 315r CCD detector.
Once entry ports are sealed, the microfluidic chip provides a solid and air-tight system in which crystals can be safely transported and stored for several months while preserving their intrinsic quality. Some protein crystals were used after 3 years !
Yes, CrystalChip can be loaded in an imager system for direct crystal visualization. The CrystalChip has dimensions of a standard microscope slide (25mm x 75mm), and will fit on any compatible insert. Please contact us at [email protected] if you need further advice.
CrystalChip has been specifically designed to prepare crystals for analysis by X-ray diffraction and has not been directly applied to cryo-EM so far. Indeed, once crystals have formed in the microfluidic chip, they are typically kept inside the chip for in situ analyses as it can be quite tricky to fish them out. Analyses that can be carried out directly in the chip include X-ray crystallography, serial crystallography and also direct optical imaging methods (fluorescence imaging, polarized light, etc) as the chip is at a standard microscope format. That being said, CrystalChip can provide a useful complementary tool to use alongside single particle cryo-EM to quickly generate X-ray crystallographic atomic models alongside cryo-EM workflows. This can be particularly useful if you want to get a more complete structural picture of a molecule, dock atomic models into a cryo-EM map or resolve small or highly dynamic structures that may present challenges for cryo-EM. Indeed, performing X-ray analyses in the chip requires very little protein solution, and the protocol is really straight-forward (you simply have to inject your protein & crystallization solutions, and all analyses are performed in situ at room temperature).
Lipidic cubic phases are unfortunately too viscous to be injected inside the microfluidic system of the chips. That being said, CrystalChip can very well be used for membrane protein crystallization. Membrane protein crystals have been successfully obtained in the chip using membrane protein solutions containing a detergent (cf paper below).
2019 | A simple and versatile microfluidic device for efficient biomacromolecule crystallization and structural analysis by serial crystallography
de Wijn R, Hennig O, Roche J, Engilberge S, Rollet K, Fernandez-Millan P, Brillet K, Betat H, Mörl M, Roussel A, Girard E, Mueller-Dieckmann C, Fox GC, Olieric V, Gavira JA, Lorber B, Sauter C. IUCrJ. 2019 Apr 19;6(Pt 3):454-464. doi: 10.1107/S2052252519003622. PMID: 31098026; PMCID: PMC6503916.
These microfluidic chips take advantage of the counter-diffusion principle. As soon as the sample and precipitant solution are loaded, a continuous gradient of concentrations is created by liquid-liquid diffusion: each substance has a higher concentration at the end where it was introduced, creating strong, opposing gradients that drive diffusion. This is made possible by the geometry of the capillary channels, providing a convection-free environment that is prone to maintaining well-defined concentration gradients for both substances (the flow is dominated by diffusion rather than convection because of the small dimensions of the channels).
Crystals formed in the CrystalChip are micron-sized, in accordance with the 80µm x 80µm channels.
A few dozens of crystals are typically required to determine a structure using CrystalChip when using synchrotron beamlines. Unlike XFELs, synchrotron beams are less intense so it is generally possible to measure a partial dataset on each crystal by collecting small rotation wedges before radiation damage causes the resolution to drop.
Although these chips have been initially developed for biomolecule crystallization, they could be very useful for resolving the structures of other types of molecules too. Relying on the counter-diffusion principle, they provide a very convenient tool to maximize your chance of finding the right conditions for crystallization while saving reagents. They can also offer a well-suited environment to flow external compounds (i.e. ligands, hit molecules, etc) on a crystallized compound.
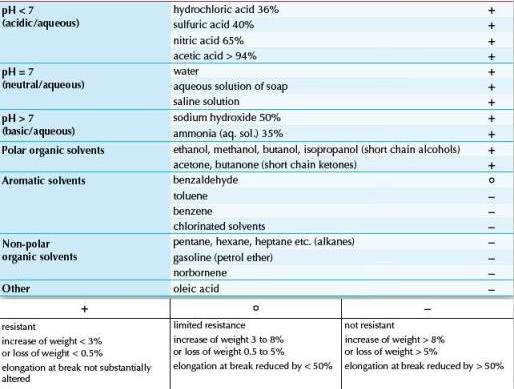
The main things to be careful about when crystallizing small molecules in the chip is the choice of the solvent, which has to be compatible with the chip material. The chip is made of a copolymer (COC), and will therefore be compatible with most organic polar solvents. Non-polar/halogenated solvents such as chloroform or dichloromethane should be avoided as they will alter the material. Dimethylformamide (DMF) has already been successfully used with the CrystalChip.
Another important parameter to take into account is the choice of the crystallization solutions. Indeed, those provided in the "screening kits" were optimized to screen for biomolecule crystallization conditions and contain a series of concentrated PEG & ammonium sulphate solutions with pH ranging from 3 to 10. Precise composition and concentration of those used for your molecule might need to be experimentally determined.
The chip is made of a copolymer (COC), and will therefore be compatible with most organic polar solvents. Non-polar/halogenated solvents such as chloroform or dichloromethane should be avoided as they will alter the material. Dimethylformamide (DMF) has already been successfully used with the CrystalChip.
Here is a table detailing the chip compatibility with different various solvents:
Yes, exact conditions at a defined location along the microchannels can be calculated using an equation governing diffusion-driven mixing (Fick’s second law) knowing the concentration of each solution loaded and the spatial coordinates along the direction of diffusion. Some collaborating labs are currently investigating whether a prediction tool could be developed for that aim.
That being said, the primary aim of this chip is to integrate all steps from biomolecule crystallization to X-ray diffraction analysis, so that you don't have to reproduce precise crystallization conditions using an alternative method. Because the capillary dimensions and the counter-diffusion mixing are highly reproducible, it's easy to retrieve similar conditions even in separate CrystalChip experiments by loading the same successful crystallant solutions in the reservoirs.
Any other questions on CrystalChip? Please contact us !
Publications about CrystalChip:
2024 | Protein crystallization and structure determination at room temperature in the CrystalChip
Petr Pachl, Léna Coudray, Romain Vincent, Léa Nilles, Hélène Scheer, Christophe Ritzenthaler, Adéla Fejfarová, Pavlína Řezáčová, Sylvain Engilberge, Claude Sauter. FEBS Open Bio, doi:10.1002/2211-5463.13932
2021 | Crystallization and Structural Determination of an Enzyme:Substrate Complex by Serial Crystallography in a Versatile Microfluidic Chip
de Wijn, R., Rollet, K., Olieric, V., Hennig, O., Thome, N., Noûs, C., Paulus, C., Lorber, B., Betat, H., Mörl, M., Sauter, J. Vis. Exp. (169), e61972, doi:10.3791/61972.
2019 | A simple and versatile microfluidic device for efficient biomacromolecule crystallization and structural analysis by serial crystallography
de Wijn R, Hennig O, Roche J, Engilberge S, Rollet K, Fernandez-Millan P, Brillet K, Betat H, Mörl M, Roussel A, Girard E, Mueller-Dieckmann C, Fox GC, Olieric V, Gavira JA, Lorber B, Sauter C. IUCrJ. 2019 Apr 19;6(Pt 3):454-464. doi: 10.1107/S2052252519003622. PMID: 31098026; PMCID: PMC6503916.
2013 | ChipX: A Novel Microfluidic Chip for Counter-Diffusion Crystallization of Biomolecules and in Situ Crystal Analysis at Room Temperature
Franziska Pinker, Mathieu Brun, Pierre Morin, Anne-Laure Deman, Jean-François Chateaux, Vincent Oliéric, Christian Stirnimann, Bernard Lorber, Nicolas Terrier, Rosaria Ferrigno, and Claude Sauter. Cryst. Growth Des. 2013, 13, 8, 3333–3340.
Webinars:
Bridging the Gap: From Microfluidic Crystals to High-Throughput Imaging
Join us in this webinar to learn more about how combining the advantages of the microfluidic format with high-throughput imaging opens exciting perspectives in protein crystallization studies.