- All Products
- SensiDeath - Ultra-sensitive human cell death assay
SensiDeath - Ultra-sensitive human cell death assay
https://www.idylle-labs.com/shop/sensideath-513 https://www.idylle-labs.com/web/image/product.template/513/image_1920?unique=7b6d0daUltra-sensitive PCR-based human cell death assay
A technology developed by Eric Lacazette, Henrik Laurell & Christian Touriol (Equipe ANUBIS, Université de Toulouse, France).
SensiDeath is a novel sensitive kit for human cell death detection. Taking benefit from qPCR high sensitivity, SensiDeath provides a simple method to detect cell death at its earliest stages by quantifying genomic DNA fragments released in the cytoplasm. Use it to get reliable measurements of apoptosis across a range of cell populations.
SensiDeath at a glance
Fast & simple
As simple as a qPCR. Lyse your cells, collect the cytosolic fraction and get results in less than 2h !
Extended dynamic range
Get reliable data and make sure to never miss out subtle changes in cell death.
Scalable
Compatible with 96-well plate format and efficient on small cell populations, SensiDeath is a convenient method for high-throughput cytotoxicity assays.
Early detection
Based on extremely sensitive cytosolic detection of genomic DNA, SensiDeath detects cell death as soon as it is initiated and up to its late phase.
How does SensiDeath work?
SensiDeath is based on a patented technology relying on the quantitation of genomic DNA released in the cell cytosol, a key process initiated upon cell death and a valuable marker of apoptosis. By amplifying genomic DNA present in isolated cytosolic fractions, you can quickly compare relative levels of apoptotic cells across a wide range of samples.
Lyse your sample
using our special lysis buffer disrupting cell plasma membrane specifically
Centrifuge
your lysate to recover the cytosolic fraction
Amplify
specific sequences present in the genome by quantitative PCR using our optimized primers
Assess cell death
that is directly proportional to cytosolic enrichment in genomic DNA
Applications of SensiDeath
Outputs:
comparative measurements of cell death levels for cytotoxic or viability assays. SensiDeath can be used as a reliable method to distinguish changes in apoptosis*.
*Check out our Results section for detailed results.
Sample types: human cells (adherent & suspension)*, tumoroids
*Check out our FAQ section for a full list of validated cell lines.
Analysis: PCR-based techniques (quantitative PCR, digital droplet PCR).
Additional resources
Kit contents of SensiDeath
All our kits include a pair of optimized primers designed to amplify a genomic repeat DNA element. They have been thoroughly selected and optimized to
combine high sensitivity, specificity and reproducibility in cell death detection.
Our kits also include a concentrated lysis buffer, developed and optimized to recover cytosolic fractions by disrupting the cell plasma membrane specifically while maintaining integrity of the nuclear membrane as well as that of other cellular organelles.
Small kit (>300 assays*)
Primer mix (20X): 300 µL
SensiDeath Lysis buffer (6X): 25 mL
Extended kit (>1000 assays*)
Primer mix (20X): 1 mL
SensiDeath Lysis buffer (6X): 85 mL
*Number of assays are given for 20 µL qPCR reactions. More assays can be carried out with each kit if working with smaller qPCR reaction volumes.
Storage: the 6X lysis buffer can be stored at 4°C for at least 1 year. Primers can be stored at 4°C for one month, or at -20°C for long-term storage.
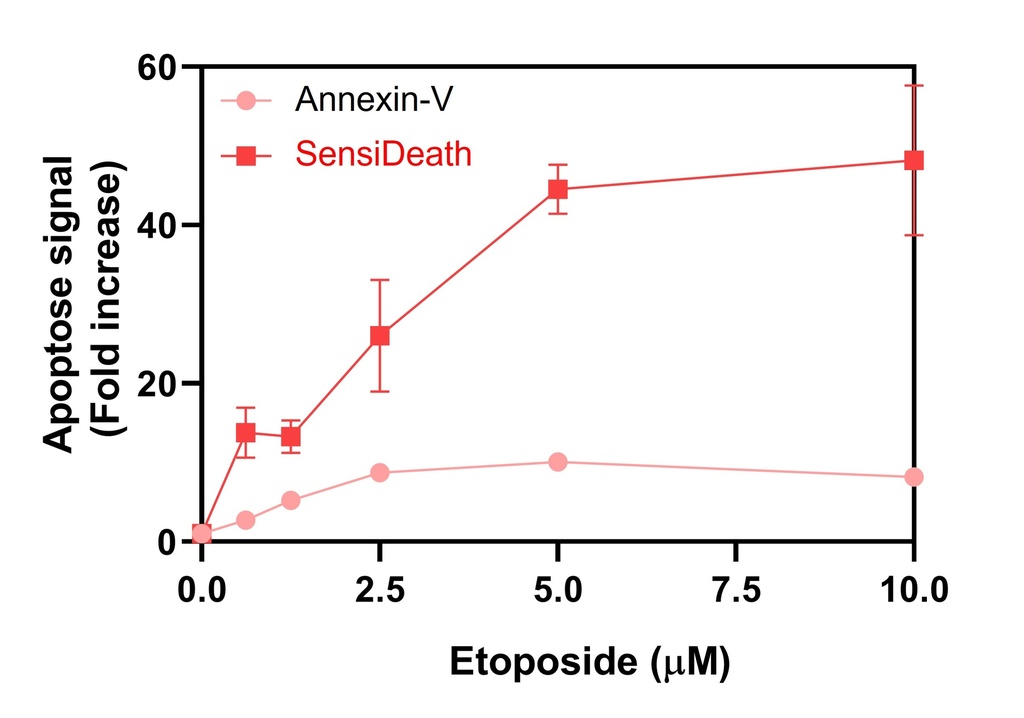
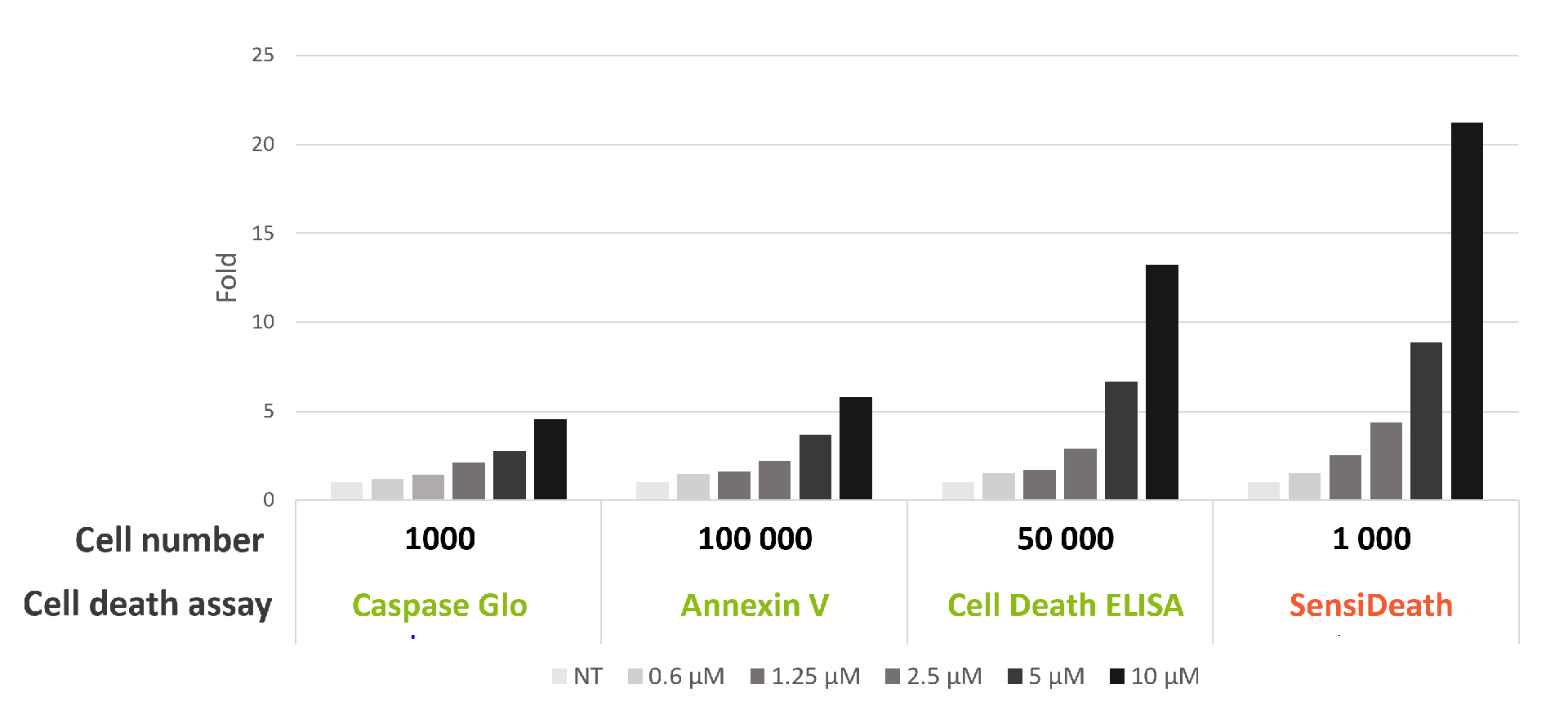
SensiDeath provides increased sensitivity in cell death detection compared to existing methods
Extended dynamic range of SensiDeath compared to existing methods
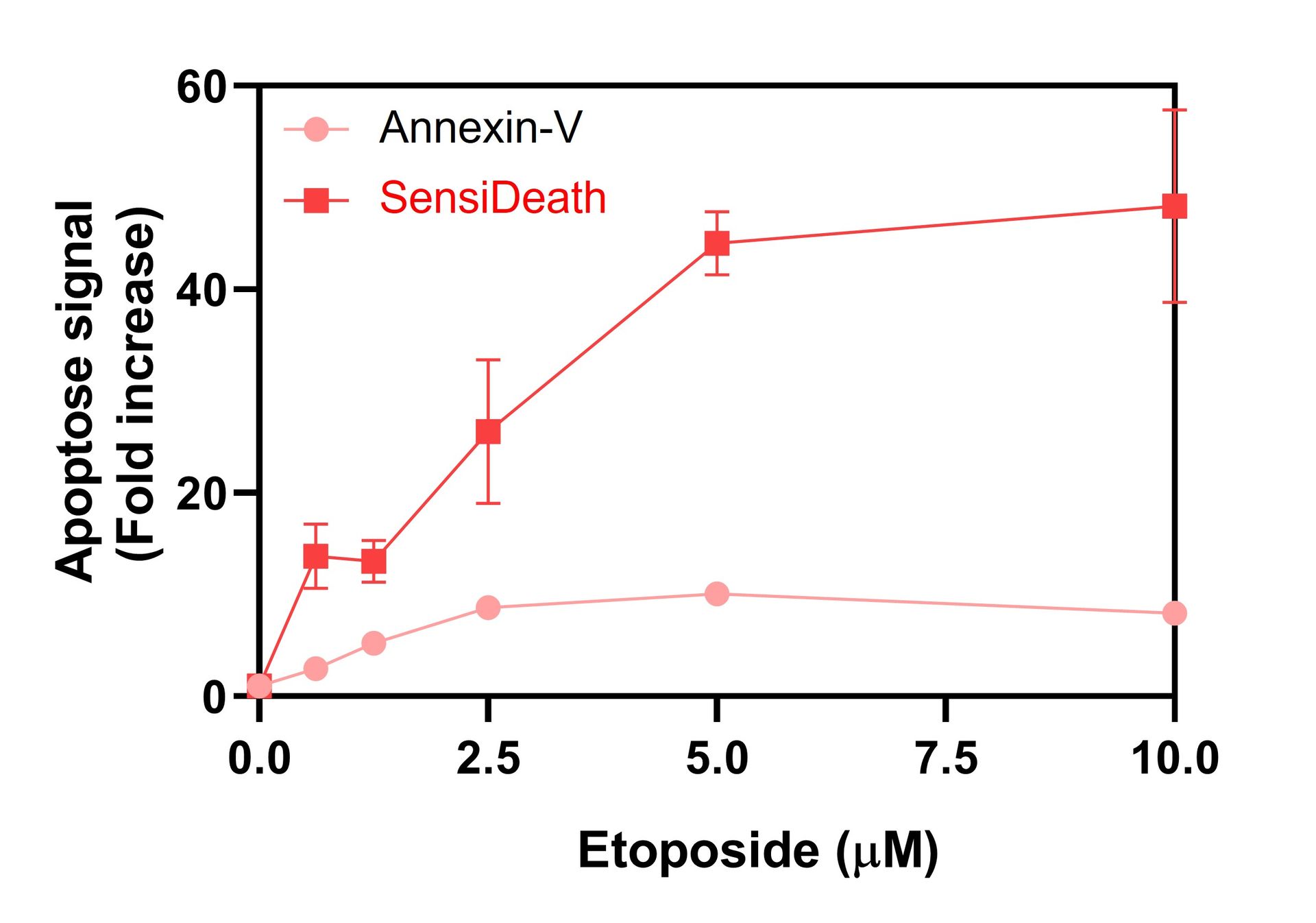
Benchmarking of SensiDeath against other common methods
How to use SensiDeath:
Click here to read and download the full SensiDeath protocol.
During cell death, fragmented genomic DNA accumulates in the cytosol. This process takes place as soon as cell death is initiated, and persists up till later stages. Using a selective lysis buffer and qPCR primers, SensiDeath allows a rapid and highly sensitive cell death quantification by detecting genomic DNA fragments released in the cytosol.
Yes, the specific primers provided in the SensiDeath kit are designed to amplify apoptotic DNA generated as soon as cell death is initiated. Several assays confirmed that SensiDeath allowed to distinguish changes in apoptosis using a variety of apoptotic inducers. Check out our Results section for detailed results.
The presence of nuclear DNA in the cytosol can be detected in early, mid and late phase apoptosis. The cytosolic concentration of nuclear DNA will be maximal in the mid-phase, but the extreme sensitivity of the SensiDeath method enables its detection in its earliest phases too.
There are several benefits in being able to catch cell death early-on.
For example, if you are interested in particular signalling pathways involved during the earliest stages of the cell death process (i.e. bcl-2). Finding cytotoxicity assays that are able to detect cell death before 16h can be tricky, making it difficult to get reliable data on early death mechanisms.
But being able to pick up cell death soon after it is initiated is not only a way to identify early processes. Something that motivated the inventors to develop a method for early detection was also very much that it simply provides more reliable data: you can be sure you detect dying cells as soon as the death process is initiated, and don't miss out even the smallest changes in cell death other methods might leave out. This can be particularly useful if you're working with complex death modulators, inducing only subtle changes in cell death levels.
The SensiDeath method relies on the amplification by qPCR of genomic DNA present in the cell cytosolic fraction. By amplifying repeated genomic sequences present in the cytosolic fraction, you can assess the relative cytosolic enrichment in genomic DNA in comparison to an untreated, negative control. This enrichment will be directly proportional to the cell death levels in your sample.
SensiDeath has been successfully used to detect cell death in a variety of human cell lines, including:
- Adherent cells: HepG2, MDA-MB-231, HeLa, SK-Hep, HEK293, HUVEC, primary fibroblasts, NK-cell, monocyte & macrophage cell lines
- Suspension cells: OCI-AML3, MOLM14, MV4-11, HL-60, THP-1, U937, etc
- 3D cell aggregates: rhabdomyosarcome-derived tumoroids
Please note that this list is non-exhaustive and
SensiDeath could be used to assess cell death in any other human cell types.
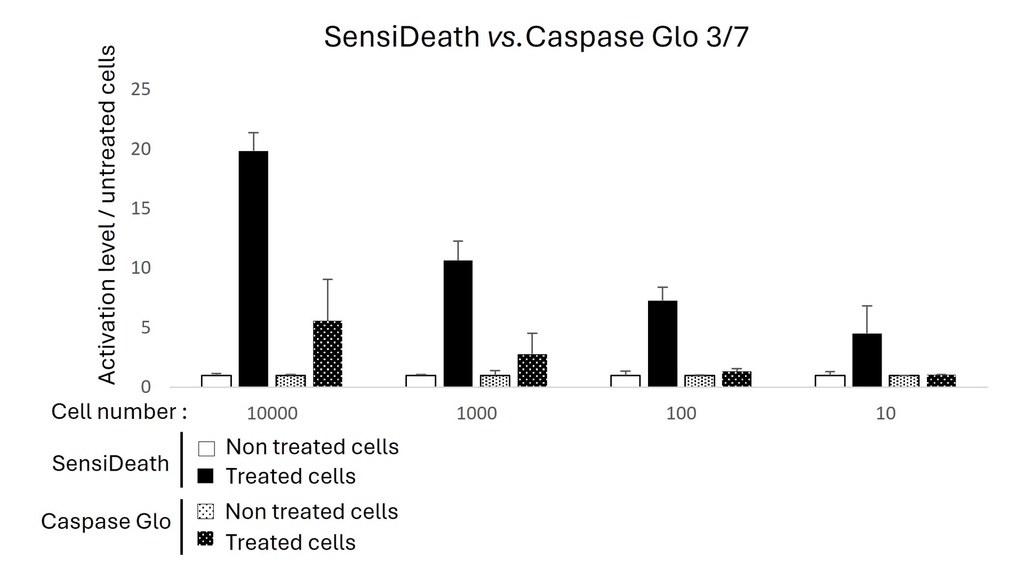
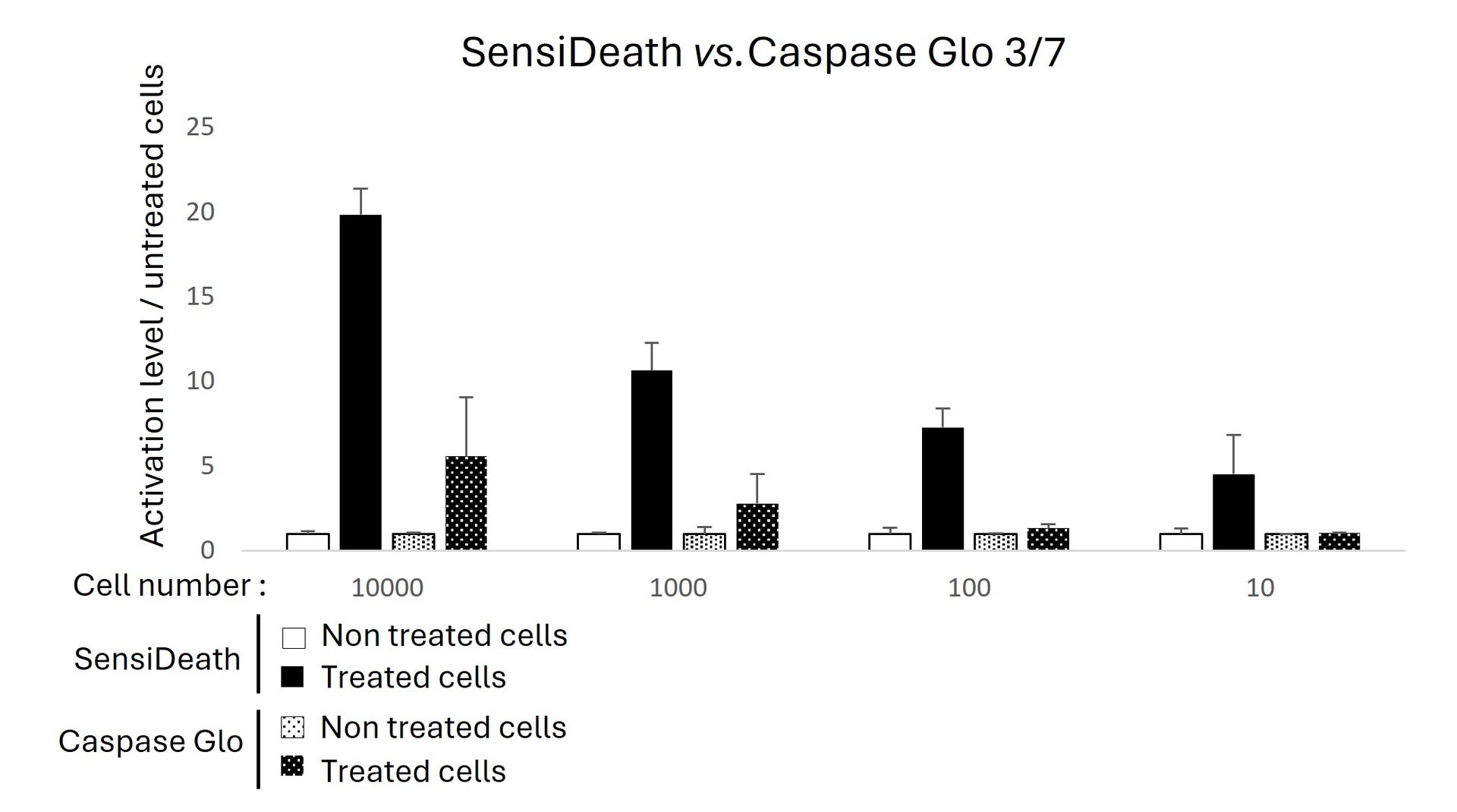
SensiDeath is perfectly well-suited for high-throughput and automated assay formats. Extracted cytosolic fractions can be analysed in 96-well or smaller plate formats. The centrifugation step can be replaced by a magnetic-bead system to purify cytosolic DNA fragments.
We provide optimized primers for amplifying a 153 bp genomic repeated element. This primer pair has been meticulously selected to ensure high sensitivity, specificity and reproducibility.
The SensiDeath kits contain a concentrated lysis buffer and optimized primers for genomic DNA amplification. The only reagent needed is nuclease-free water and any SYBR Green qPCR mix. In terms of equipment, you need a simple benchtop centrifuge for sample preparation and a thermocycler for performing the qPCR. You do not need any sophisticated equipment.
After lysate centrifugation, nuclear fractions are pelleted at the bottom of the tube. A few microliters of the supernatant are collected for analysis, thus excluding the risk of nuclear contamination.
Yes, nucleases are efficiently inhibited by the lysis buffer. Extracted cytosolic fractions can be kept at -20°C for months before being assayed. This is particularly useful when performing kinetics studies, or simply to save time by assaying multiple samples in parallel.
Yes, the lysis buffer can be added directly to cell culture medium as long as its final concentration is 1X.
Yes, the qPCR amplification signal will reflect the basal level of apoptosis in the control cell population.
No, our lysis buffer provided in the kit has been specifically optimized to disrupt the plasma cell membrane while leaving the nuclear membrane, as well as that of all other organelles, perfectly intact. Therefore, there is no risk of retrieving any mitochondrial DNA in the isolated cytosolic fractions.
The SensiDeath lysis buffer provided in the kit has been specifically developed for destructing the cell plasma membrane while keeping the membrane of nuclei and other organelles intact, therefore allowing the specific retrieval of the cytosolic fraction. In addition, its components have been optimized not to interfere with subsequent qPCR reactions. Therefore, we do not recommend using other lysis buffers as we cannot guarantee the accuracy of the obtained cell death measurements.
Yes, the SensiDeath lysis protocol preserves the integrity of the nucleus and other organelles (mitochondria, ER and Golgi) while extracting the cytosolic fraction. Therefore, it is entirely possible to recover the organelle and nucleoplasm contents to carry out complementary assays from the same lysate. This has been successfully done using detergent-based sequential subcellular fractionation for Western blot analysis.
Indeed, some DNA can also be present in the cell cytosol during other processes, especially mitosis. Some tests have been carried out to check whether changes in the cell cycle phases might skew the obtained cytotoxicity data. Non-apoptotic cells were labelled with propidium iodide, and sorted using flow cytometry according to their cell cycle phase (G0/G1, S or G2/M). The SensiDeath protocol has been performed on these samples, and results showed similar qPCR signals across the different samples demonstrating that changes in the cell cycle phase did not affect the obtained data.
Lysis, when performed according to the protocol's guidelines (15 minutes at room temperature), should not affect the nuclear membrane. This has been tested and validated by the inventors of the method, who meticulously optimized the composition and concentration of the different components of the lysis buffer to ensure that the nuclear membrane remains intact under the described conditions.
If your results are reproducible and consistent with the treatments applied, it is unlikely that the nuclear membrane has been compromised. However, if in doubt or if modifying the lysis buffer concentration and/or incubation duration, it is always possible to verify the integrity of the nuclear membrane using various methods (for example, by using an antibody targeting a nuclear protein like lamin A/C or histones).
The primers provided are simple oligos: any kind of qPCR mix and compatible detection systems can be used as long as they are based on SYBR Green qPCR. The primers provided do not include any probes for Taqman.
SensiDeath is based on a technology patented by Henrik LAURELL, Eric LACAZETTE and Christian TOURIOL (n°WO2020188216A1). The full patent is available online: https://patents.google.com/patent/WO2020188216A1/en?oq=WO+2020%2f188216